
hotline:
17715390137
Tel/Wechat:
18101240246 (Technology)
0512-68565571
Email:mxenes@163.com (Sales Engineer)bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online

 【Research Background】
【Research Background】
In sustainable energy systems, supercapacitors represent highly efficient energy storage devices for unbalanced power needs. According to different charge storage mechanisms, supercapacitors can be divided into electrochemical double-layer capacitors (EDLC) and rhenium capacitors. In EDLC, an electrochemical double layer is formed on the electrode surface, and a polarizing solvent between the ions and the electrode serves as a dielectric medium. Rhenium capacitors store energy through intercalation or electrosorption and Faraday charge transfer through a redox reaction. The theoretical capacitance of intercalated rhenium capacitors can be extremely high, so it is more attractive. In order to achieve high capacitance, some early attempts focused on intercalated 赝 capacitor materials, Nb2O5, V2O5, MoS2, and VS2. These materials show an open layered structure that can achieve rapid migration and containment of ions in active materials. However, most fluorene-capacitive oxides or sulfides have limited electrical conductivity, resulting in poor electrochemical performance. Therefore, in large-scale applications such as power grids, new types of highly conductive intercalation electrode materials are urgently needed.
[Achievement Profile]
Recently, Professor Xiaohui Wang of the Chinese Academy of Sciences and Professor Quan-Hong Yang‘s group from Tianjin University published a research paper titled Interlayer engineering of Ti3C2Tx MXene towards high capacitance supercapacitors in the internationally renowned academic journal Nanoscale. How Engineering Helps Approaching the Limit. The inter-layer engineering design creates a wide and uniform inter-layer spacing, thereby providing a "highway" for rapid ion diffusion and providing "trucks" (redox-active sites) on such "highways" to accelerate charge Transfer makes high capacitance possible. According to this concept, the capacitance of Ti3C2TxMXene made by annealing in ammonia gas has been greatly improved, and it has excellent rate performance and cycle performance. The overall performance of the well-designed MXene is superior to all other plutonium capacitor electrodes.
[Picture and text guide]
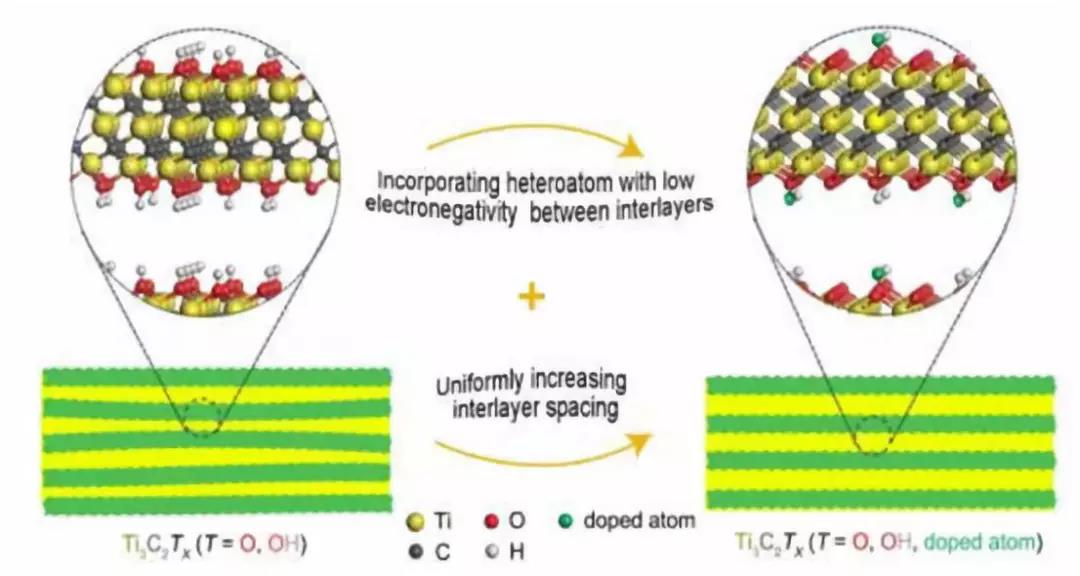 Figure 1. Schematic diagram of the middle layer of Ti3C2Tx MXene used to achieve high capacitance
Figure 1. Schematic diagram of the middle layer of Ti3C2Tx MXene used to achieve high capacitance
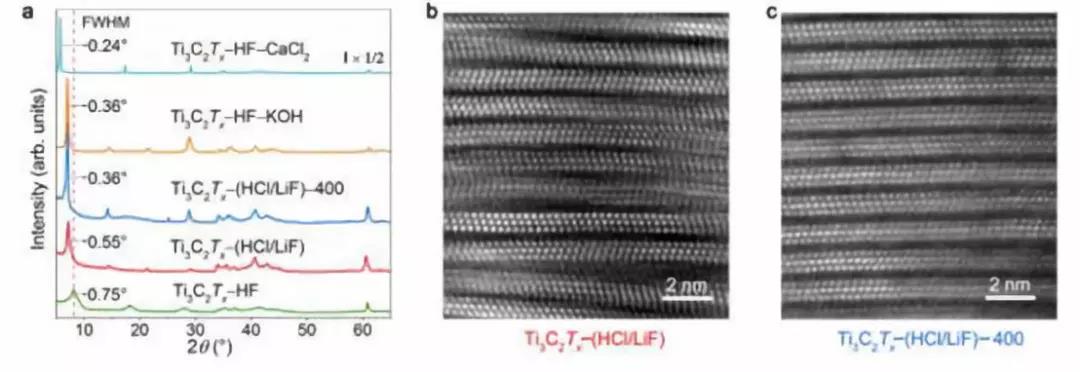 Figure 2. Structural characterization of Ti3C2TxMXenes after different treatments. (A) XRD pattern of Ti3C2TxMXene. The MAX phase was etched in HF and HCl / LiF solutions to prepare Ti3C2Tx-HF and Ti3C2Tx- (HCl / LiF); Ti3C2Tx- (HCl / LiF) -400 was heated by heating Ti3C2Tx- (HCl / LiF). Ti3C2Tx-HF-KOH and Ti3C2Tx-HF-CaCl2 are treated in KOH and CaCl2 solutions, respectively (b, c) (b) Ti3C2Tx- (HCl / LiF) and (c) Ti3C2Tx- (HCl / LiF) -400 HAADF image
Figure 2. Structural characterization of Ti3C2TxMXenes after different treatments. (A) XRD pattern of Ti3C2TxMXene. The MAX phase was etched in HF and HCl / LiF solutions to prepare Ti3C2Tx-HF and Ti3C2Tx- (HCl / LiF); Ti3C2Tx- (HCl / LiF) -400 was heated by heating Ti3C2Tx- (HCl / LiF). Ti3C2Tx-HF-KOH and Ti3C2Tx-HF-CaCl2 are treated in KOH and CaCl2 solutions, respectively (b, c) (b) Ti3C2Tx- (HCl / LiF) and (c) Ti3C2Tx- (HCl / LiF) -400 HAADF image

Figure 3. Capacitance performance of Ti3C2Tx electrodes prepared by different preparation methods in 1 mol L-1 H2SO4 electrolyte. (a) CV curve at a scan rate of 20 mV s-1, and (b) Mass capacitance at different scan rates. At different scan rates, the capacitance of Ti3C2Tx-(HCl / LiF) -400 is significantly larger than the capacitance of other electrodes (c) CV curve of Ti3C2Tx-(HCl / LiF) -400 at a scan rate of 2 to 100 mV s-1 (D) Capacitance retention of Ti3C2Tx-(HCl / LiF) -400 electrode under constant current cycling at 10 A g-1, internal graph constant current cycling curve
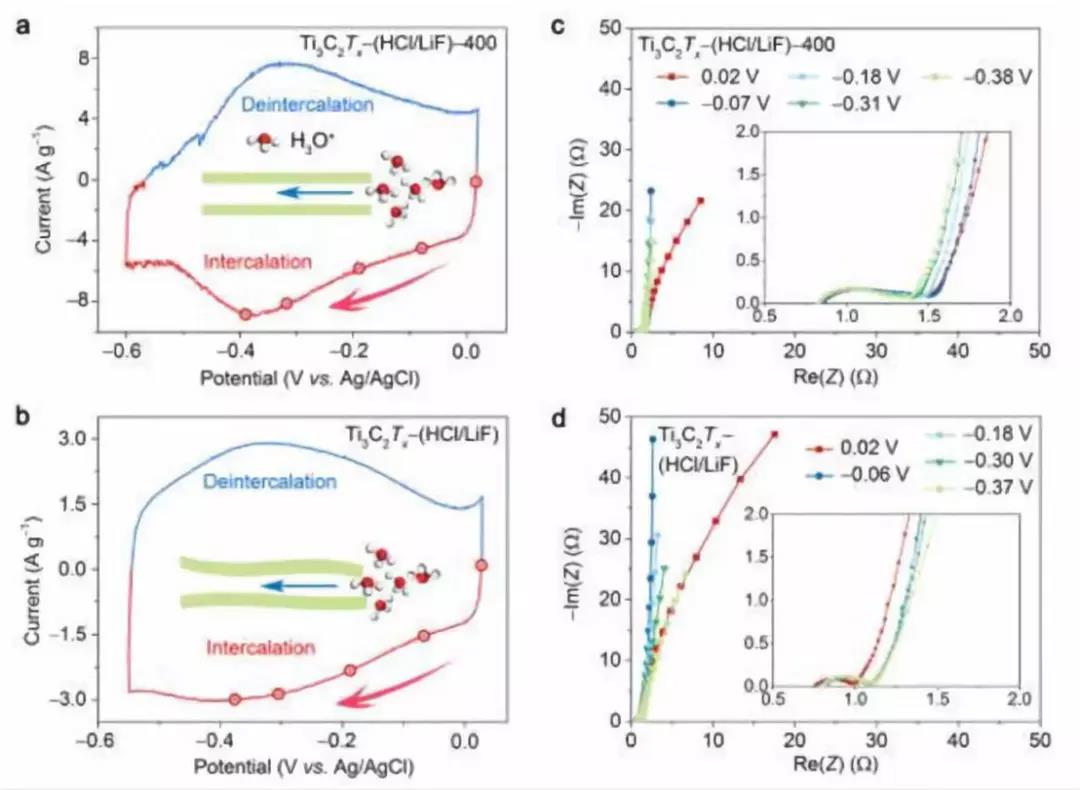 Figure 4. In-situ EIS spectra of Ti3C2Tx- (HCl / LiF) and Ti3C2Tx- (HCl / LiF) -400 electrodes (a) Ti3C2Tx- (HCl / LiF) -400 and (b) Ti3C2Tx- (HCl / LiF) electrode CV curve. Schematic diagram of hydrogen ions embedded in the MXene layer. (C, d) on the intercalation (red filled ball) branches of (c) Ti3C2Tx-(HCl / LiF) -400 and (d) Ti3C2Tx- (HCl / LiF) electrodes at different voltages vs. Ag / AgCl EIS data (Nyquist plot)
Figure 4. In-situ EIS spectra of Ti3C2Tx- (HCl / LiF) and Ti3C2Tx- (HCl / LiF) -400 electrodes (a) Ti3C2Tx- (HCl / LiF) -400 and (b) Ti3C2Tx- (HCl / LiF) electrode CV curve. Schematic diagram of hydrogen ions embedded in the MXene layer. (C, d) on the intercalation (red filled ball) branches of (c) Ti3C2Tx-(HCl / LiF) -400 and (d) Ti3C2Tx- (HCl / LiF) electrodes at different voltages vs. Ag / AgCl EIS data (Nyquist plot)
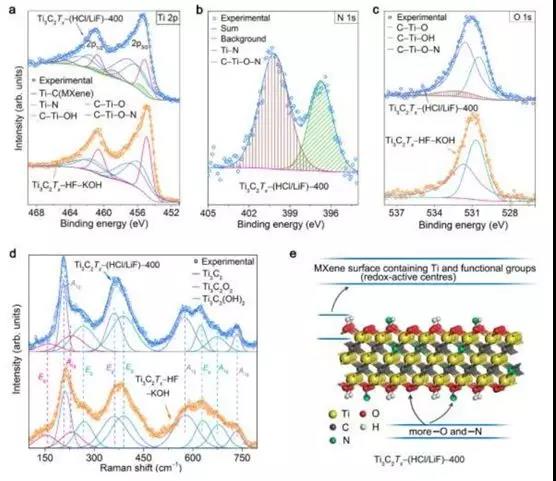 Figure 5. Chemical composition and morphological characterization of Ti3C2Tx-(HCl / LiF) -400 and Ti3C2Tx -HF-KOH. (A) Ti 2p XPS spectrum, (b) N 1sXPS spectrum, (c) O 1s XPS spectrum, (d) Raman spectrum and selected Lorentz fit of Raman band (e) Ti3C2Tx- Chemical composition of (HCl / LiF) -400
Figure 5. Chemical composition and morphological characterization of Ti3C2Tx-(HCl / LiF) -400 and Ti3C2Tx -HF-KOH. (A) Ti 2p XPS spectrum, (b) N 1sXPS spectrum, (c) O 1s XPS spectrum, (d) Raman spectrum and selected Lorentz fit of Raman band (e) Ti3C2Tx- Chemical composition of (HCl / LiF) -400
[Summary of this article]
We take Ti3C2Tx MXene as an example and propose the concept of interlayer engineering to achieve high capacitance. Designing in such a way that at the same time creating an open and uniform interlayer spacing and incorporating heteroatoms with lower electronegativity between the layers makes high capacitance possible. The wide and uniform interlayer spacing provides a "highway" for rapid ion diffusion, and makes the surface of the more active Ti3C2Tx skeleton easier to enter the electrolyte; meanwhile, doped heteroatoms with lower electronegativity between the intermediate layers More "truck" -redox active sites can be provided on the "highway" for charge transfer. More trucks on the highway lead to high permittivity. Following the concept of interlayer engineering and annealing in an ammonia atmosphere, the capacitance of 570 F g-1 exceeds the capacitance of reported electrode materials. The feasible sandwich engineering verified in this paper provides guidance for the design of MXene-based electrode materials with enhanced capacitors. This principle can also be applied to other two-dimensional layered materials.
Literature link:
DOI: 10.1039 / c9nr08960h

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd 京ICP备16054715-2号 |