
hotline:
17715390137
Tel/Wechat:
18101240246 (Technology)
0512-68565571
Email:mxenes@163.com (Sales Engineer)bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online


【Research Background】
2D metal carbide / nitride (MXenes) is an excellent electrochemically active pseudocapacitor material. Multilayer MXenes are similar to graphene, but with larger layers that have both surface and functional groups, making them easy to disperse in water and carrying out a series of reactions without losing their conductivity. The larger interlayer distance of MXenes can intercalate larger ions to form composite materials, such as graphene, metal oxides, transition metal sulfides, and silicon. These composite electrodes can be maintained after thousands of cycles. Excellent capacity at higher rates. Since 2011, MXene‘s research in the field of energy storage has grown exponentially. Therefore, for researchers new to the field of MXenes, it is necessary to systematically understand the current research status. Recently, Mark A. Bissett and Suelen Barg of the University of Manchester in the United Kingdom published a review article entitled A Review of MXene-Based Anodes for Metal-Ion Batteries in Battery, the internationally renowned academic journal in the field of batteries.
[Picture and text guide]

Figure 1. The main differences between graphene anodes and MXene anodes.

Table 1. Application of pure MXene electrode in lithium battery.

Figure 2. Sandwich structure of Na0.23TiO2 / Ti3C2 electrode and its electrochemical performance. Schematic diagram of ion and electron transport of Li4Ti5O12-Ti3C2TX.

Figure 3. Synthesis process and electrochemical performance test of MoS2 / Ti3C2 @ C nanocomposite.

Table 2. Application of transition metal sulfide and MXene composite electrodes in lithium batteries.

Figure 4. Ti3C2TX and CNTs form a composite by microwave radiation, and its electrochemical performance is tested.

Table 3. Application of carbon, silicon and MXene composite electrodes in lithium batteries.

Figure 4. Synthesis of Ti3C2TX and Si composite electrode and testing of its electrochemical performance.

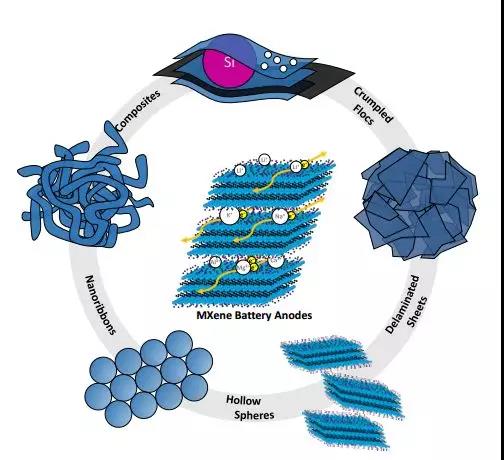
Figure 5. MXene colloid solution as a precursor, nanoribbons, wrinkled particles and hollow spheres are formed in different ways.

Figure 6. Application of MXene-based electrodes in sodium ion batteries.

Figure 7. Electrochemical performance test of rGO @ Ti3C2TX foam.
[Summary and Prospect]
Because of the existence of transition metals, higher rate performance and long-term cycle stability cannot usually be guaranteed with higher capacity. Although MXene is denser and more expensive than graphene, when MXenes are used as flexible conductive substrates or multifunctional When used as a binder, it has great potential in the application of lithium batteries. In order to fully utilize the entire MXene nanosheet, it is necessary to ensure a sufficient interlayer distance, usually by layering or expanding the interlayer distance to ensure the plating of the metal and reduce the barrier potential of the deep adsorption site, especially for larger sodium ions Battery with potassium ion. Although porous structures have been proven to effectively increase capacity, our understanding of pore size, shape, and spatial frequency is far from deep enough. These are the most important aspects of our balance between porosity and physical examination capacity and loss of electronic conductivity. Weight. Research on printed MXenes has made some progress recently, which may help us to study the microstructure of MXene electrodes.
Literature link:
http://dx.doi.org/10.1002/batt.201900165

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd 京ICP备16054715-2号 |