
hotline:
17715390137
Tel/Wechat:
18101240246 (Technology)
0512-68565571
Email:mxenes@163.com (Sales Engineer)bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online

腾龙国际上下分客服【521006914微信QQ同步】腾龙官方网站(www.TL9043.com)腾龙国际客服24小时在线,【公司直属客服】【公司直属开户】【大额无忧】支持视频验证现场。
With the rapid development of portable wearable electronic devices, flexible, safe and high energy density energy storage devices have gradually become a research hotspot. Lithium metal secondary batteries have become very promising candidates because of their ultra-high theoretical specific capacity. However, the problem of lithium dendrites caused by the uneven deposition of metallic lithium, as well as the volume change problem of the lithium anode during cycling, seriously affects the safety and service life of the battery. In addition, due to the rapid consumption of lithium resources, the issue of utilization of lithium anodes also needs attention. The development of ultra-thin flexible self-supporting carriers can effectively solve the above problems. Two-dimensional transition metal carbon / nitride (MXene) has huge application potential in the field of energy storage due to its high conductivity, graphene-like structure, rich functional groups, and easy film formation. However, the general mechanical properties of MXene thin films are poor, and MXene is prone to re-stacking and other problems during cycling, which limits its practical application in flexible metal lithium anodes. Therefore, it is extremely valuable to explore and develop MXene-based ultra-thin flexible self-supporting electrodes.
【Job Introduction】
Recently, Prof. Guo Zaiping, University of Wollongong , Australia, and Associate Researcher Ye Huan, and Prof. Feifei Cao from Huazhong Agricultural University jointly designed an interlocking topology. By self-assembly of MXene nanosheets and nanocellulose (CNF), combined with electrochemical deposition Process, successfully prepared ultra-thin flexible metal lithium composite anode (MXene @ CNF / Li). The obtained metal lithium composite negative electrode is matched with the flexible positive electrode to assemble a flexible full battery, and exhibits excellent electrochemical performance. Relevant achievements were published in the international top journal Nano Energy . The first authors of the paper are Wang Caoyu, a graduate student of Huazhong Agricultural University and Zheng Zijian, associate professor of Hubei University.
The results show that MXene and CNF can self-assemble through hydrogen bonding , forming MXene @ CNF microspheres in the process of rotary evaporation. The formed MXene @ CNF microspheres and the surplus MXene sheets overlap each other to form an interlocking topology, enhance the interaction force between the interfaces, and significantly improve the mechanical strength and flexibility of the MXene @ CNF film. In addition, the sandwich structure of MXene sheet and MXene @ CNF microspheres can effectively prevent MXene from stacking again and improve the lithium ion transmission kinetics. The expanded interlayer spacing can accommodate more metal lithium, and realize the deposition of higher area capacity lithium. The abundant lithium-philic functional groups on the surface of MXene can form strong mutual adsorption with lithium, and induce uniform nucleation of metal lithium and subsequent dendrite-free growth. Thanks to the above advantages, the constructed lithium composite negative electrode can achieve an ultra-thin thickness of ~ 25 μm, and the assembled symmetrical battery can stably cycle for more than 1300 hours at a current density of 0.5 mA / cm 2 . In addition, the ultra-thin MXene @ CNF / Li composite lithium negative electrode and flexible self-supporting LFP @ CNF composite positive electrode are matched to assemble a flexible full battery. The flexible full battery is cycled nearly 100 times at 0.2 C, and the capacity retention rate can be maintained at about 95.25%, showing good cycle stability. This work not only provides an idea for the simple and large-scale preparation of ultra-thin, flexible and self-supporting MXene-based films, but also broadens the practical application of MXene-based materials in the field of flexible energy storage.
【Content expression】
As shown in Figure 1, MXene and nanocellulose (CNF) self-assemble through hydrogen bonding to form an interlocking topology film. The flexible MXene @ CNF / Li lithium composite anode constructed with this film as a carrier exhibits high mechanical strength and toughness. The flexible MXene @ CNF / Li lithium composite negative electrode can maintain good film characteristics without bending after repeated bending-recovery.
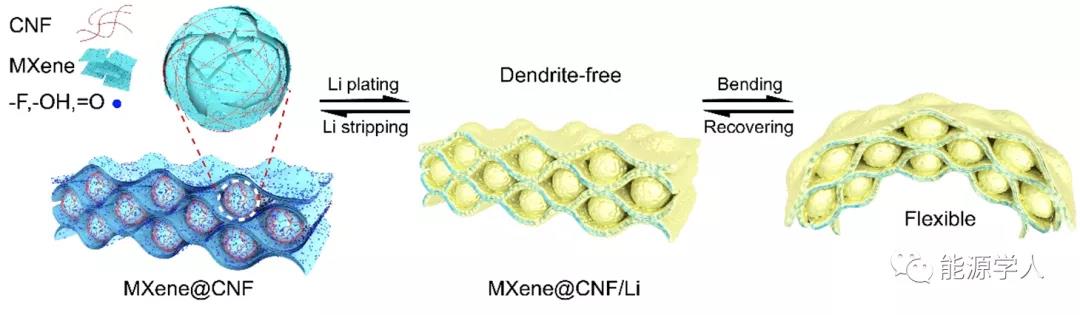
Figure 1. Schematic diagram of MXene @ CNF topology.
By etching with hydrochloric acid and lithium fluoride, MXene nanosheets with a size of 1 to 2 μm were obtained (Figure 2a-c). After the suspension containing the MXene nanosheets and the CNF colloid solution are thoroughly mixed, the MXene @ CNF flexible film with an interlocking topology is obtained by the rotary evaporation technology . The MXene @ CNF flexible film exhibits excellent mechanical properties and flexibility, and can be folded into various shapes (Figure 2d). Scanning electron microscope (SEM) characterization showed that the thickness of the MXene @ CNF film was around 20 μm (Figure 2e). The film is composed of MXene nanosheets and MXene @ CNF microspheres overlapping each other to form an interlocking topology (Figure 2f-g). In order to explore the structure and composition of the microspheres, the author carried out transmission electron microscope (TEM) image characterization and EDX element analysis. The results show that the elements of Na, C, F, and Ti are evenly distributed on the microspheres (Figure 2h-l), indicating that the microspheres are assembled from layers of nanosheets.

Figure 2. (a) TEM image of MXene nanosheets. (B) HRTEM picture of MXene nanosheets, and (c) corresponding lattice spacing. (D) MXene @ CNF origami optical photo. (E), (f) SEM image of MXene @ CNF film cross section, and (g) planar SEM image. (H) TEM image of MXene @ CNF microsphere, and (i ~ l) corresponding EDX element energy spectrum image.
The formation mechanism of MXene @ CNF microspheres is shown in Figure 3. From a thermodynamic point of view, both MXene and cellulose surfaces are rich in -O, -OH, -F groups, they tend to interact to form intramolecular hydrogen bonds, self-assemble to form layered micelles, and reduce the enthalpy change of the system D H. In the process of rotary evaporation, the layered micelles are curled to form spherical micelles under the action of drum granulation. Under the action of hydrogen bonds, the layered micelles and spherical micelles are further assembled by layers to form MXene @ CNF Microspheres. Finally, a sandwich structure composed of layered micelles and microspheres is obtained. This unique topology helps to enhance the mechanical strength and flexibility of the membrane material.
Compared with pure MXene film, MXene @ CNF composite film has the following advantages : (1) The interlocking topology between the sheet and the microspheres can enhance the interaction between the interfaces and improve the mechanical properties of the flexible metal lithium anode; (2 ) Ultra-thin self-supporting MXene @ CNF / Li composite lithium negative electrode is conducive to the rapid transmission of lithium ions and electrons, ensuring high lithium utilization rate and good rate performance; (3) Rich polar functional groups on the surface can be used as lithium lipophiles The nucleation site induces uniform deposition of lithium metal; (4) This method is simple and low in cost, which is convenient for future popularization and application.
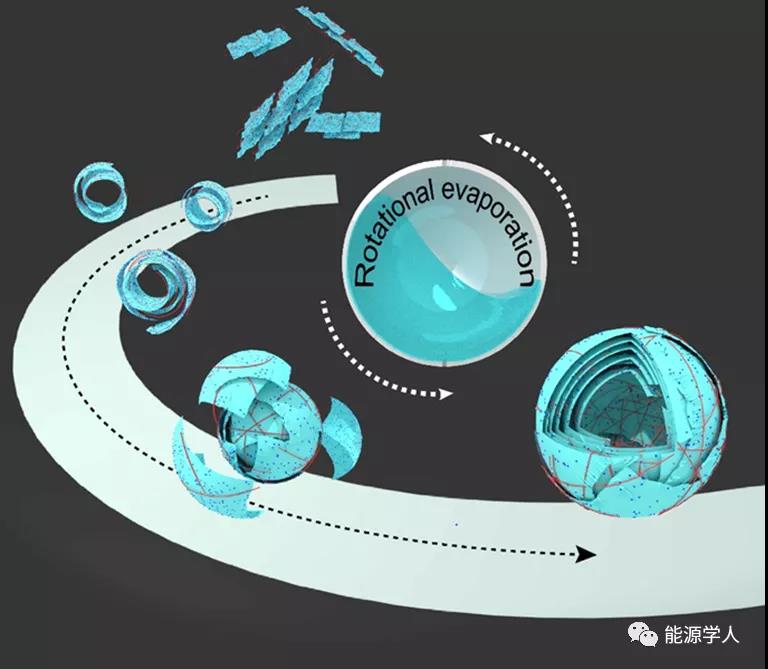
Figure 3. Schematic diagram of MXene @ CNF microsphere formation.
First-principles theoretical calculations show that there are strong binding capabilities between the rich polar functional groups on the surface of MXene and lithium atoms, which can induce uniform nucleation and growth of metallic lithium during lithium deposition-precipitation (Figure 4a). The authors studied the morphology difference of the deposited metal Li on the flat Cu, MXene film and MXene @ CNF surface at the same current density (0.5 mA / cm 2 ) by scanning electron microscopy (SEM) . Due to the unevenness of the microscopic surface of the copper foil, needle-like lithium can be detected on the surface of the copper foil. Thanks to the induction of lithium functional groups by the polar functional groups on MXene, petal-like Li deposits are regularly dispersed on the MXene film. Li also showed a petal-like shape on MXene @ CNF.
After depositing 0.5 mA h / cm 2 lithium, some microspheres have petal-like spots (Figure 4b, f), and dendritic lithium is not visible on the surface and inside. When the deposition capacity of metallic lithium was increased to 1 mA h / cm 2 , the surface of the MXene @ CNF film was almost completely covered with petal-like lithium (Figure 4c, g). As the deposited lithium capacity further increased to 2 mA h / cm 2 , petal-like lithium remained good, and the internal hierarchical structure began to fill and become dense (Figure 4d, h). Because the MXene @ CNF film has a large interlayer spacing, it can provide sufficient space for the deposition of metal lithium and slow down the volume change problem of metal lithium anodes. Therefore, the structure of the thin film remains stable after the lithium metal is extracted (Figure 4e, i).
On further by ToF-SIMS to MXene @ CNF / Li anode were characterized, studies have shown that, Li elements in the spatial distribution of the film F., Uniform spatial distribution of the elements O, showed that metal lithium in well distributed on the surface, and effectively It is contained in the interlayer of MXene @ CNF film, which is consistent with the above SEM image. This result also reflects the strong interaction between the polar functional group on the surface of MXene and Li, which can be used as a deposition site to induce the uniform deposition of metallic lithium on the thin film to avoid the formation of metallic lithium dendrites.

Figure 4. (a) The binding energy between Li atoms and Ti 3 C 2 and Ti 3 C 2 F 2 . SEM images of lithium metal deposited on the MXene @ CNF film (b) 0.5 mA h / cm 2 , (c) 1 mA h / cm 2 , (d) 2 mA h / cm 2 , and corresponding cross-sections (f), ( g), (h) SEM images. (E), (i) SEM image after the lithium metal is released from MXene @ CNF. The element distribution of (j) Li, (k) O, and (l) F (2 mA h / cm 2 ) on MXene @ CNF / Li negative electrode was characterized by ToF-SIMS .
The uniform deposition of lithium metal on MXene @ CNF is conducive to improving the coulombic efficiency and cycle life of lithium metal anodes (Figure 5). Studies have shown that at an area capacity of 2 mA h / cm 2 and a current density of 0.5 mA / cm 2 , the initial coulombic efficiency of lithium metal on MXene @ CNF reaches 82.5%, the cycle is> 200 cycles, and the efficiency can be stabilized at about 98.9% . The constructed MXene @ CNF / Li symmetrical battery has a stable cycle of more than 1300 h and a voltage of about 50 mV at a current density of 0.5 mA / cm 2 .
In order to further study the bending resistance of the MXene @ CNF / Li flexible composite negative electrode, the authors removed the Cu / Li negative electrode and the MXene @ CNF / Li negative electrode after stable cycling from the battery and conducted dozens of bending tests. The results show that after the folding test, the part of the lithium metal deposited on the surface of the copper foil is peeled off or even detached from the surface of the copper foil, and due to the metal fatigue effect, the surface of the copper foil is creased or even broken. The MXene @ CNF / Li flexible negative electrode has good mechanical properties, and the structure of the composite lithium negative electrode remains intact. Recycle the negative electrode after the bending test, the Cu / Li negative electrode quickly fails, and the MXene @ CNF / Li flexible negative electrode can still cycle normally. This shows that the flexible composite negative electrode can be applied to flexible devices.
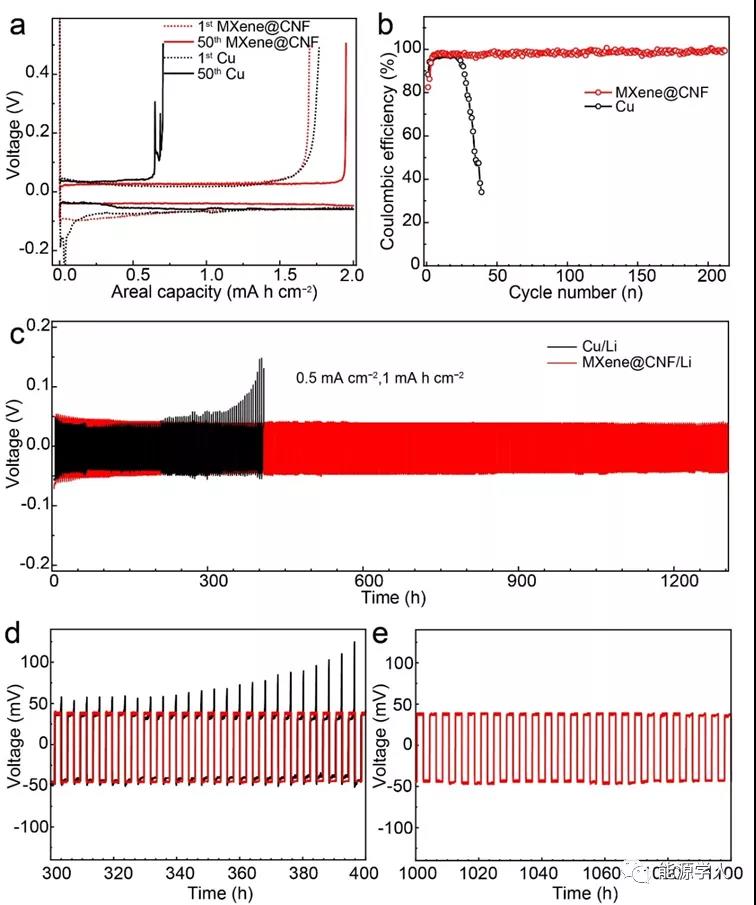
Figure 5. (a) At a current density of 0.5 mA / cm 2 and a lithium deposition amount of 2 mA h / cm 2 , the constant current charge and discharge curves of MXene @ CNF and Cu intercalation / delithiation of lithium correspond to ( b) Coulomb efficiency. (C) Symmetrical battery test of MXene @ CNF / Li and Cu / Li anodes at a current density of 0.5 mA / cm 2 , and (d) partially enlarged views of 200-400 h, (e) 1000-1100 h.
In order to test the applicability of MXene @ CNF / Li composite negative electrode, the author matched it with a high-load lithium iron phosphate positive electrode to construct a full battery. In the charge-discharge voltage range of 2.0-4.0 V, the MXene @ CNF || LFP full battery shows a higher specific capacity and a longer cycle life than the Cu || Li full battery. Thanks to the above-mentioned topological advantages of the MXene @ CNF / Li composite negative electrode, the MXene @ CNF || LFP full battery exhibits good rate performance. At a rate of 2 C, it can exert a reversible specific capacity of 120 mAh / g. The author further assembled flexible full cells with MXene @ CNF / Li negative electrode and LFP @ CNF flexible positive electrode to explore its feasibility in constructing flexible devices. Studies have shown that at a rate of 0.2 C, the MXene @ CNF / Li || LFP @ CNF flexible full battery can exert a specific capacity of more than 150 mA h / g, the cycle is close to 100 cycles, and the capacity retention rate can be maintained at 95.25%, The application prospect of MXene @ CNF / Li composite negative electrode in flexible energy storage devices is shown.
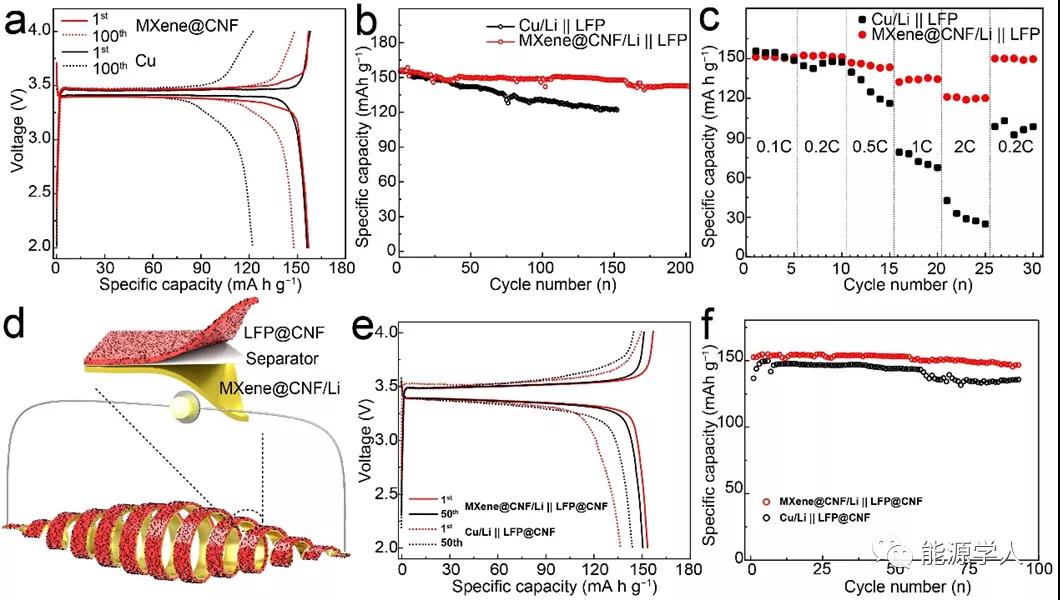
Figure 6. (a) MXene @ CNF / Li || LFP, Cu / Li || LFP full-cell constant current charge and discharge curve at 0.2 C and (b) corresponding cycle performance, (c) rate performance. (D) MXene @ CNF / Li || LFP @ CNF flexible full battery schematic. (E) MXene @ CNF / Li || LFP @ CNF, Cu / Li || LFP @ CNF flexible full battery constant current charge and discharge curve at 0.2 C and (f) corresponding cycle performance.
【to sum up】
An ultra-thin (~ 25 μm), flexible, self-supporting Ti 3 C 2 T x -MXene @ CNF thin film based on the design concept of interlocking topology is reported . Thanks to the interlocking microstructure between the MXene sheet and the MXene @ CNF microspheres, the MXene @ CNF film exhibits good mechanical strength and flexibility, and the enlarged interlayer spacing ensures high surface capacity and rate performance of the lithium anode. The rich lipophilic functional groups on the surface of MXene show a strong interaction with Li, which can induce the uniform deposition of Li metal and avoid the growth of lithium dendrites. The composite lithium anode circulates 250 cycles at a current density of 1 mA / cm 2 and still maintains 98.9% Coulomb efficiency. The composite lithium negative electrode shows an extremely long cycle life (0.5 mA / cm 2 cycle 1300 h). The flexible full battery assembled based on the flexible MXene @ CNF / Li composite negative electrode and the flexible self-supporting LFP @ CNF positive electrode shows good long-cycle stability, with about 100 cycles and a capacity retention rate of 95.25%. This work not only provides an idea for the simple and large-scale preparation of ultra-thin, flexible and self-supporting MXene-based films, but also broadens the practical application of MXene-based materials in the field of flexible energy storage.
Cao-Yu Wang, Zi-Jian Zheng, Yong-Qiang Feng, Huan Ye * , Fei-Fei Cao * , and Zai-Ping Guo * , Topological Design of Ultrastrong MXene Paper Hosted Li Enables Ultrathin and Fully Flexible Lithium Metal Batteries, Nano Energy , 2020, DOI: 10.1016 / j.nanoen.2020.104817
Source of information: Energy scholar

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd 京ICP备16054715-2号 |