
hotline:
17715390137
Tel/Wechat:
18101240246 (Technology)
0512-68565571
Email:mxenes@163.com (Sales Engineer)bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online

Researcher Zhao Xin of Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences realized the first controlled synthesis of structural isomers of covalent organic frameworks (COFs) through polycondensation conditions. The resulting two isomers exhibit different gas / vapor adsorption properties and chemical stability It can be converted from one isomer to another under certain conditions.
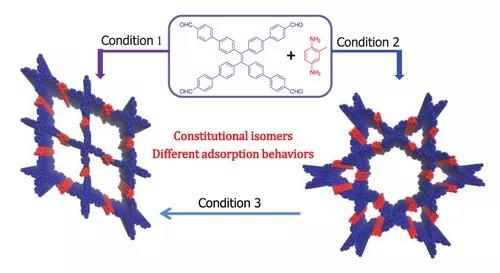
"Isomerization" is a common phenomenon in organic chemistry. Isomers often exhibit different physical and chemical properties. In recent years, the phenomenon of isomerization has also been found in some emerging materials. For example, in metal organic frameworks (MOFs), the same composition primitives can form different structures of “frame isomers (different structures) through different connection methods. Construct) ". Most of these isomers were obtained accidentally in experiments, and it is difficult to achieve controlled synthesis. As pure organic similar structures of MOFs, covalent organic frameworks (Covalent Organic Frameworks, COFs) are a type of crystalline organic porous polymers formed by a regular grid structure connected by organic elements through covalent bonds. Structure determines performance, and its conjugated skeleton and highly ordered nanopore structure give them many applications from adsorption, separation, catalysis to optoelectronic materials. Similar to MOFs, COFs should also have isomerism, but it is limited by factors such as bond formation and crystallization process in the construction of COFs. Previously, there was only one case of three-dimensional COF isomers with the same framework structure and different interpenetration degrees ( Interpenetrating isomerism) selective preparation was reported by Wang Wei‘s research group of Lanzhou University and Sun Junliang‘s research group of Peking University ( J. Am. Chem. Soc. 2018 , 140 , 6763.), while the controlled synthesis of COF structural isomer It has not been realized yet.
Zhao Xin‘s research group of Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences focuses on how to realize the controlled synthesis of COF structural isomers. Based on the D 2h and C 2 symmetry primitive combination previously developed by the group to construct the first heteropore COF work ( J. Am. Chem. Soc. 2014 , 136 , 15885.), the combination of these two symmetry primitives In theory, two frame structures can be formed. One is composed of quadrangular pores (uniform pore COF), and the other is composed of periodic distribution of triangular and hexagonal pores (double pore COF). This system is selective A good foundation has been established for the synthesis of COF structural isomers.
Based on the selection of the above monomer structure, the research group successfully achieved the first controlled synthesis of COF structural isomers by changing the solvent conditions of the polymerization reaction (Figure 1). Using aqueous acetic acid as a catalyst and regulator, polymerization in mesitylene-dioxane can obtain a uniform pore COF structure, while using n-butanol-o-dichlorobenzene as a solvent can obtain cage-like double pore COF.
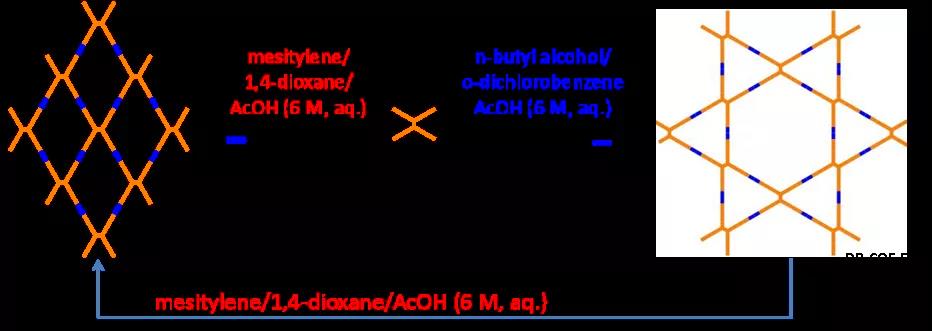
figure 1
The infrared and solid nuclear magnetic data of the two COFs obtained are almost identical, but show different x-ray powder diffraction peaks, indicating that they have the same chemical composition but different framework structures and are structural isomers of each other. Its structure was analyzed by x-ray powder diffraction combined with theoretical simulation (Figure 2), and further confirmed by the pore size distribution data based on the nitrogen adsorption and desorption experiments (Figure 3a: uniform pore COF, the distribution at 21.9 Å corresponds to the diameter of the quadrilateral pore; Figure 3b: Double-hole COF, the distributions at 12.0 and 42.2 Å correspond to the diameters of triangular and hexagonal holes, respectively).
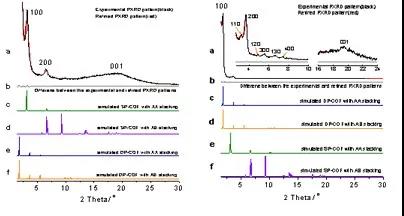
figure 2
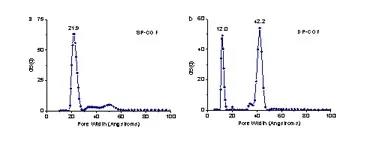
image 3
The property study found that the two COF isomers exhibit different chemical stability and also exhibit different gas adsorption properties. Uniform pore COF isomers show the adsorption characteristics of conventional microporous materials, while dual pore COF isomers show In addition to the staged adsorption and responsiveness to hexane vapor (Figure 4), further studies indicate that the latter‘s unique adsorption properties come from changes in the frame structure caused by its hierarchical pore structure and guest inclusion.
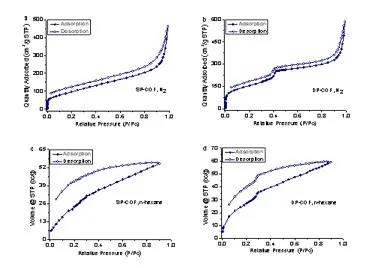
Figure 4
Finally, the author realized the complete conversion from the double pore isomer to the homogeneous pore isomer by co-heating the double pore isomer suspension with the C 2 symmetric monomer 2-methyl-p-phenylenediamine. This research is the first to realize the controlled synthesis of structural isomers of COF, expand the selective preparation of structural isomers to the field of COF, and show that different isomers have different physical and chemical properties, which is positive for revealing the formation mechanism of COFs. Meaning also helps deepen the understanding of heterogeneous phenomena. This research was supported by the National Natural Science Foundation of China, the Shanghai Municipal Science and Technology Commission and the Chinese Academy of Sciences‘ Strategic Leading Science and Technology Project (Category B). The work was published in the second issue of CCS Chemistry 2020 in the form of Communication.
Article details:
A Study on Constitutional Isomerism in Covalent Organic Frameworks: Controllable Synthesis, Transformation, and Distinct Difference in Properties
Rong-Ran Liang, Fu-Zhi Cui, Ru-Han A, Qiao-Yan Qi & Xin Zhao
Original link: https://doi.org/10.31635/ccschem.020.201900094
Citation: CCS Chem. 2020, 2, 139–145
Source of information:

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd 京ICP备16054715-2号 |