
hotline£º
17715390137
Tel/Wechat£º
18101240246 (Technology)
0512-68565571
Email£ºmxenes@163.com £¨Sales Engineer£©bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online

¡¾Research Background¡¿
Increasingly, the development of cheap and clean energy has become an urgent demand in today¡®s society. Can for use of renewable energy is systemic problems, particularly horizontal load balance. Batteries and electrochemical capacitors are the most widely used electrochemical energy storage systems. Many researchers are committed to achieving improved performance in different fields, such as power grids and electric vehicles. For example, super capacitors can achieve rapid charge and discharge, which is very important for the practical application of renewable energy.
Supercapacitors have different charge storage mechanisms. Electric double layer capacitors (EDLCs) store energy through a double electron layer formed at the electrode / electrolyte interface. EDLCs can stably achieve millions of charge-discharge cycles, thanks to its illegal Faraday charge storage method without structural changes. Based on the understanding of the energy storage mechanism in terms of atomic size, the preparation of electrodes with high specific surface area is an effective strategy to increase capacitance. The other is called a pseudocapacitor, which stores charge through a fast Faraday redox reaction on the surface. Its matrix is divided into many types, including underpotential deposition, quantum capacitance, redox capacitance, and intercalated pseudocapacitance. Its performance is between EDLCs and lithium-ion batteries, which is very suitable for applications of 10 s ~ 10 min.
As an emerging 2D material, MXenes exhibits cationic intercalation behavior in aqueous electrolytes and has a rectangular-like cyclic voltammetry curve. However, in non-aqueous electrolytes, MXenes exhibits pseudocapacitive behavior, with highly distorted, rectangular-like cyclic voltammetry curves. To develop high-performance MXene-based capacitors, it is important to understand the capacitance behavior and pseudocapacitance behavior of MXene electrodes.
¡¾Achievement Introduction¡¿
Recently, Dr. Yasunobu Ando of Kyoto University in Japan published a research paper titled: Capacitive versus Pseudocapacitive Storage in MXene in the internationally renowned academic journal A dvanced Functional Materials . The study believes that from the perspective of electronic state, hydration can prevent MXene It is coupled with the orbit of intercalated ions, and then forms a double electron layer and capacitance behavior. However, once the ions are partially dehydrated and adsorbed on the surface of MXene, due to the coupling effect of the ions with MXene, especially with the surface functional groups, electron transfer will cause pseudocapacitive behavior.
¡¾Graphic introduction¡¿
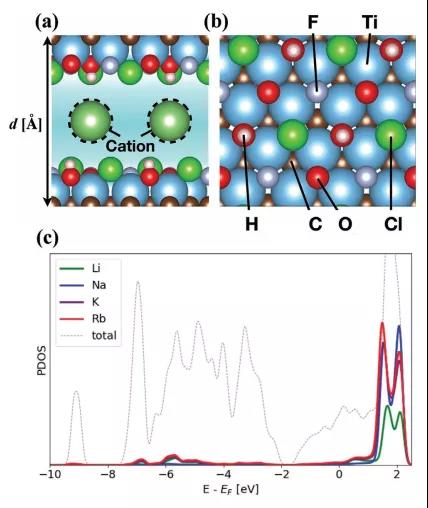
Figure 1. Simulation model of intercalated ions and PDOS.
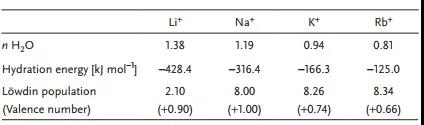
Table 1. Simulation results for various intercalated ions of MXene.
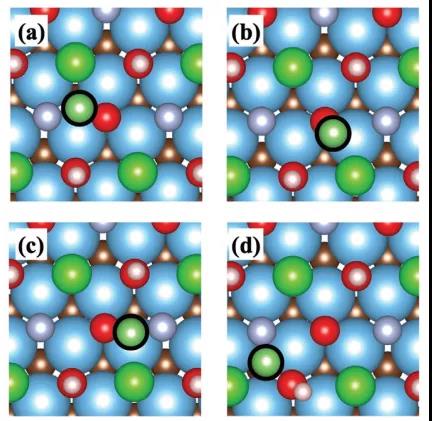
Figure 2. Optimal sites of lithium atoms at the MXene electrode considering hydration
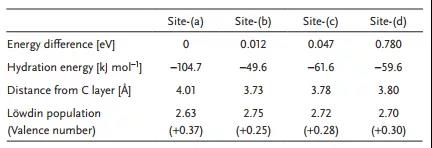
Table 2. Simulation results for MXene lithium adsorption optimization sites.
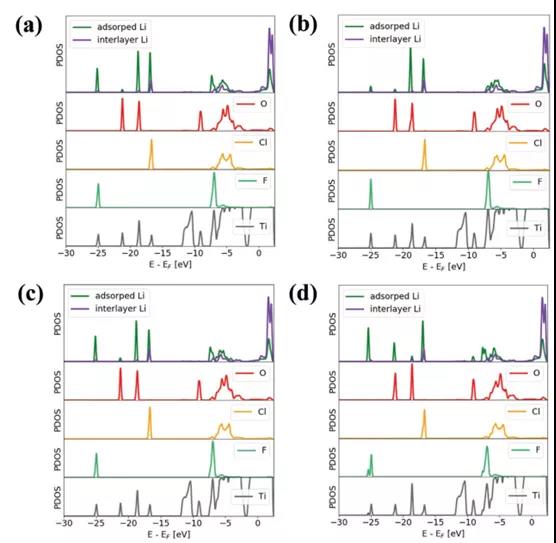
Figure 3. PDOS of lithium atoms and surface functional groups.
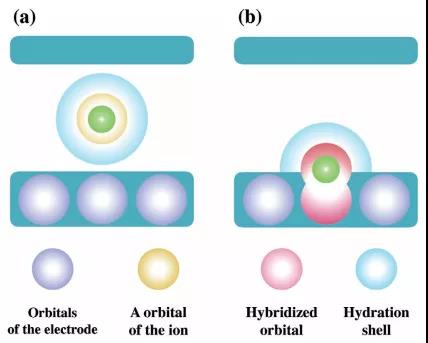
Figure 4. Schematic diagram of MXene electrode capacitance and pseudocapacitance behavior.
¡¾Summary of this article¡¿
This paper explores the charge storage mechanism of MXenes through 3D-RISM DFT composite simulation. When the fully hydrated ion intercalation enters the interlayer of MXene, the charge separation is retained and a double electron layer is formed in the interlayer region. However, for some partially dehydrated ions directly adsorbed on the surface of MXene, due to the coupling effect of ions with MXene, especially with the surface functional groups, redistribution of charge occurs. Electron transfer counteracts the potential difference generated at the electrode / electrolyte interface, and thus has pseudocapacitive behavior. Regardless of whether the MXene electrode is a capacitor or a pseudocapacitor behavior, it depends on the potential difference generated at the electrode / electrolyte interface and the reaction with intercalated ions and surface functional groups.
Literature link:
DOI: 10.1002 / adfm.202000820
Source:

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd ¾©ICP±¸16054715-2ºÅ |