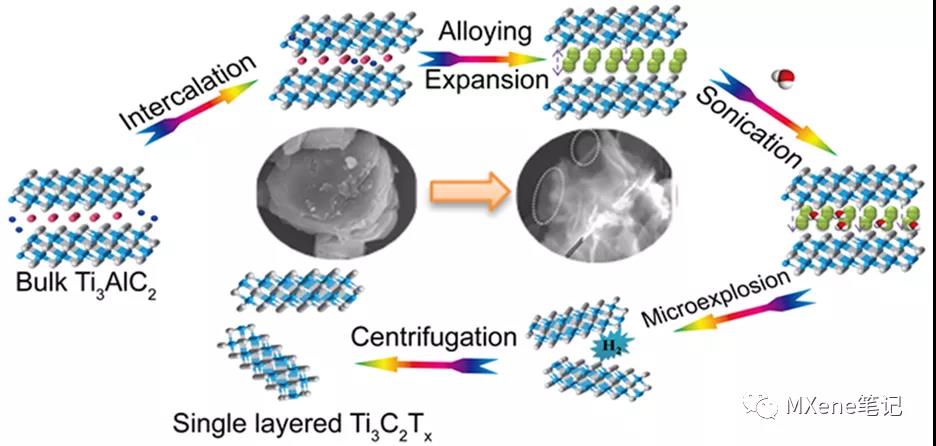
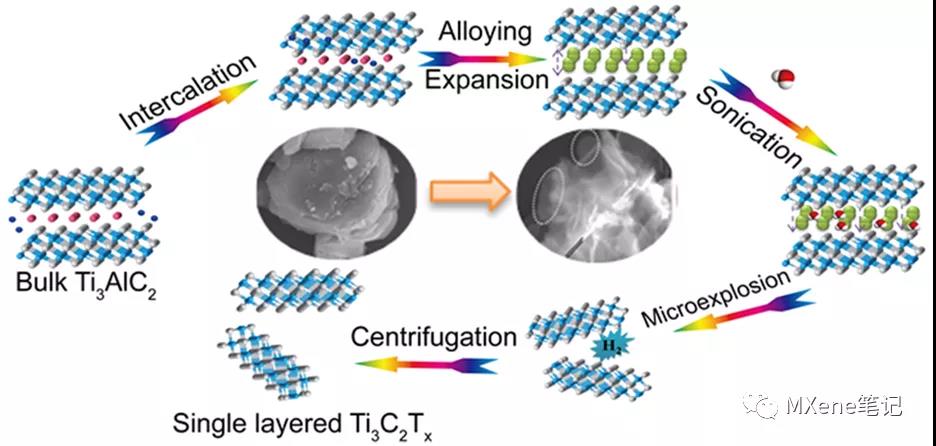
Lithiation-expansion-micro-explosion mechanism for the preparation of fluorine-free MXene materials
QQ Academic Group: 1092348845
Detailed
Research Background
Because of the unique in nature, two -dimensional material MXene study by the widespread attention, involving a number of areas. MXene preparation is MXene basic research will continue to introduce and share MXene preparation.
Selective Lithiation−Expansion−Microexplosion Synthesis of Two-Dimensional Fluoride-Free M X ene
Zemin Sun, Mengwei Yuan, Liu Lin, Han Yang, Caiyun Nan, Huifeng Li, Genban Sun, *
and Xiaojing Yang *
ACS Materials Lett. 2019, 1, 628−632 .
https://pubs.acs.org/doi/10.1021/acsmaterialslett.9b00390
brief introduction
In the conventional acid etching process, the toxicity and harm of the etching liquid limit large-scale production and application. It is very urgent to develop a harmless and effective MXene synthesis scheme. Beijing Normal University Yang Xiaojing and others have developed a simple and effective fluorine-free preparation method , which can directly prepare a single layer or a few layers of Ti3C2Tx based on the controlled lithium insertion - alloy - expansion micro-explosion mechanism . In this process, lithium ions are intercalated into the Al layer and form an Al-Li alloy under the action of the electric field . At the same time, the Al-Al layer will expand. Then put the lithiated MAX into water, and the Al-Li layer will react with water to generate hydrogen. As the micro-explosion process proceeds in an aqueous solution, the material will fall off into nanosheets, and single-layer or few-layer MXene nanosheets will be prepared with higher yields. In addition, the etching process is carried out in a reagent without fluorine, the main reagent is water, which effectively avoids the introduction of toxic liquids. Further, all the MAX phase materials whose A layer can be used as negative electrode materials for lithium ion batteries can be stripped to prepare MXene materials by this method .
Graphic guide

Figure 1. Schematic of the lithiation intercalation−expansion−microexplosion process.

Figure 2. (a) Ti3AlC2 and Ti3C2Tx powder. (B) X-ray diffraction patterns of Ti3AlC2 and Ti3C2Tx. (C) Raman spectra of Ti3AlC2 and Ti3C2Tx. (D) − (f) XPS spectra of Ti3AlC2 and Ti3C2Tx.

Figure 3. SEM images of (a) Ti3AlC2 and (b) Ti3C2Tx. (C) TEM and (d) HRTEM images of Ti3C2Tx (inset of SAED of Ti3C2Tx). (E) AFM image and (f) the relevant height profiles.

Figure 4. (a) CV curves of the Ti3C2Tx electrode in 6 M KOH aqueous solution. (B) Galvanostatic charge / discharge curves of the Ti3C2Tx electrode at different current densities. (C) Gravimetric specific capacitances versus current densities for the Ti3C2Tx electrode. (d) Cycling performance of the Ti3C2Tx electrode at a current density of 5 A / g. The inset shows the first and last five GCD curves.
Source: MXene notes
- Previous: Quick View-Oxidation o
- Next: Advanced Materials | A


 mxene academic
mxene academic