Background introduction.
Due to the lack of lymphatic reflux in tumors, the retention of tumor interstitial fluid will lead to the aggravation of tumor interstitial pressure (TIP), resulting in unsatisfactory tumor penetration in nanomedicine. It is the main inducement of tumor recurrence and metastasis. In this paper, a kind of "nano-lymphoid" based on pyroelectric catalysis is designed to reduce the hint of enhancing tumor penetration and treatment. The photothermal treatment and decomposition of tumor interstitial fluid irradiated by NIR-II laser are realized, and the hint of enhancing tumor penetration is reduced. At the same time, reactive oxygen species produced in the process of pyroelectric catalysis will further damage deep tumor stem cells. The results showed that "nano-lymphoid" alleviated 52% of TIP, enhanced tumor penetration, and effectively inhibited tumor proliferation (93.75%) and recurrence. The findings of this paper suggest a reasonable reduction of TIP in order to enhance tumor infiltration and improve treatment, which is of great significance for drug delivery.
Second, guided reading of picture and text.
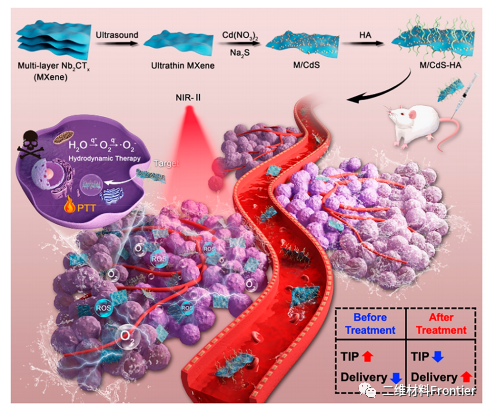
Figure 1.
Thermoelectric catalysis of nanometer lymphoid (M/CDSHA) synthesis and reduction of TIP to enhance tumor permeability, PTT and.
Hydrodynamic therapy.
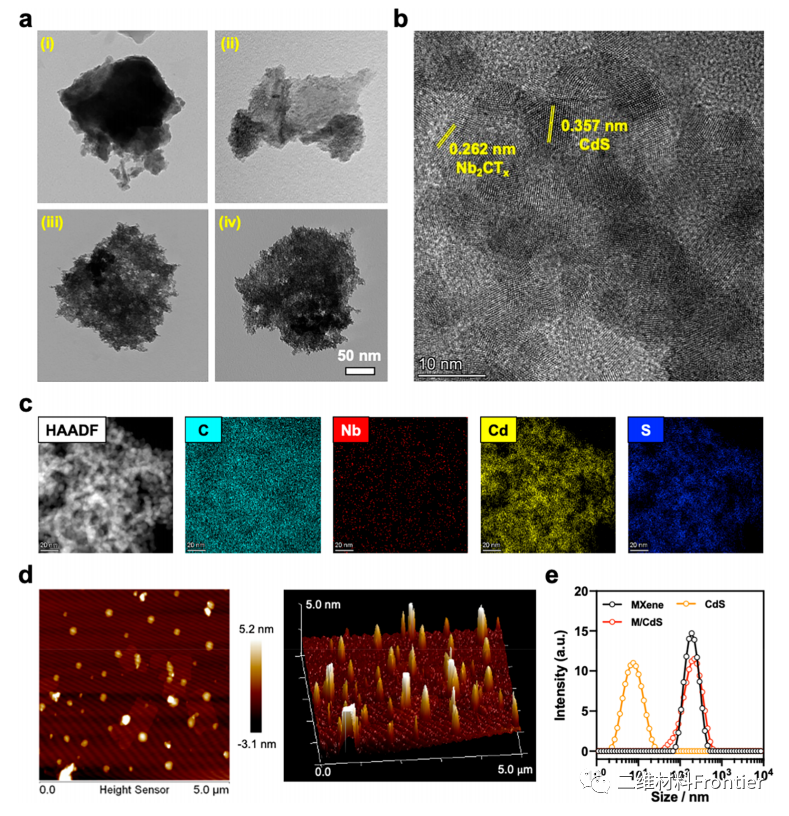
Figure 2.
(a) TEM images of multi-layer MXene (I), ultra-thin MXene (ii), M/CdS (iii) and M/CdS-HA (iv). (B) HRTEM image of M/CdS. (C) STEM-HAADF images and element mapping of M/CdS. (d) AFM image of M/CdS. (e) size distribution of MXene, CDs and M/CdS.
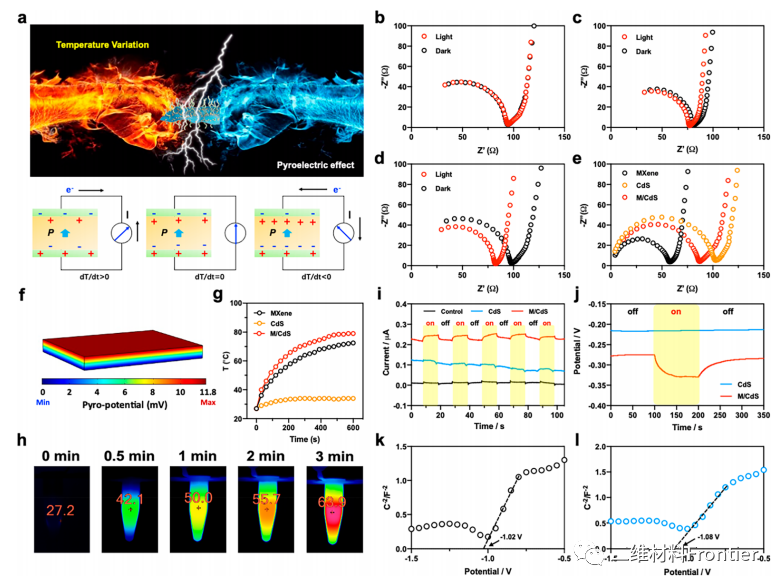
Figure 3.
(a) A schematic diagram of the relationship between pyroelectric effect and temperature change (dt/dt) and pyroelectric current (I). EISNyquist (b) CDs, (c) MXene and (d) M/CdS and 1064nm laser irradiated images (1.0W/cm2). (e) EIS Nyquist diagram of cadmium sulfide, MXene and M/CdS under laser irradiation. (F) COMSOL finite element simulation of the thermal potential of cadmium sulphide (¦¤ T = 50 ¡ãC). (G) photothermal effects of cadmium sulfide, MXene and M/CdS. (h) ITI (3min) of M/CdS irradiated by laser. (I) the thermal current of cadmium sulfide and M / cadmium sulfide under laser irradiation. (J) the thermoelectric potential of cadmium sulfide and M/CdS under laser irradiation. (K) MXene and (l) Mott-Schottky curve of cadmium sulfide.
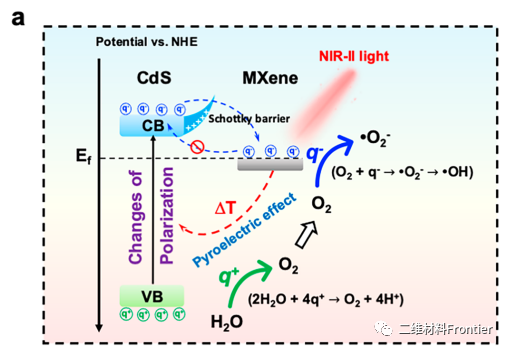
Figure 4.
Water splitting of M/CDS and pyroelectric catalysis diagram of ROS formation.
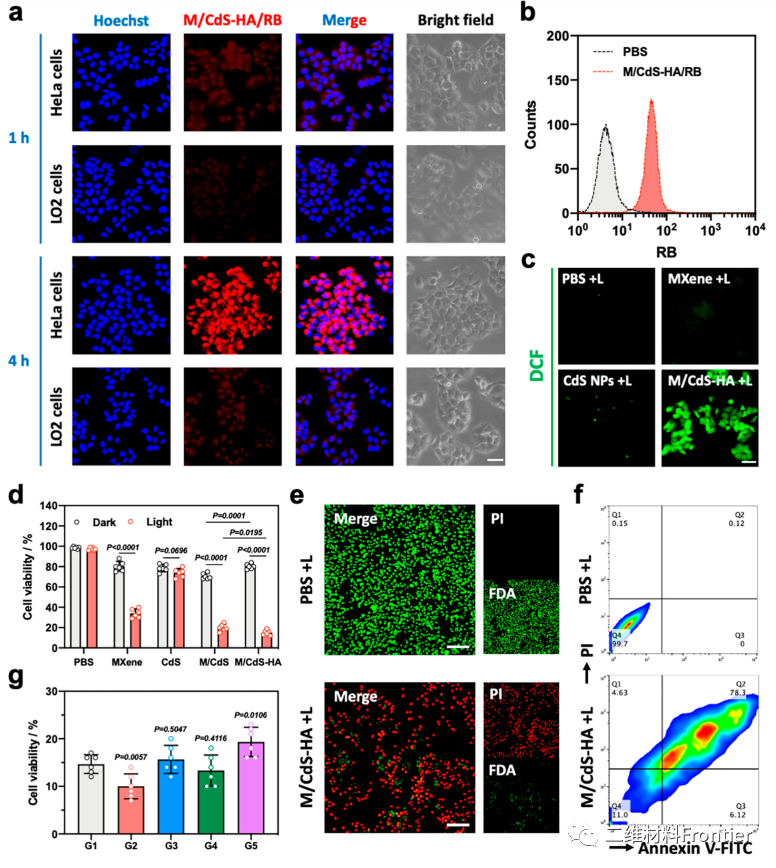
Figure 5.
(a) the fluorescence images of HeLa and LO2 cells were processed with M/CdS-HA/RB for 1 hour and 4 hours (scale bar = 20 ¦Ì m). (B) FCM analysis of HeLa cells treated with PBS and M/CdS-HA/RB. (C) in vitro ROS+L, MXene+L, CDS NPs+L and M/CdS+L-HA+L of PBS+L tested by DCFH-DA (proportional strip = 20 ¦Ì m). (d) viability of HeLa cells exposed to dark and 1064nm PBS, MXene, CDS, M/CdS and M/CdS-HA laser irradiation. (e) HeLa cells were stained with FDA/PI treated with PBS+L and M/CDS-HA+L (proportional strip = 100 ¦Ì m). (F) using PBS+L and M/CdS-HA+L. (G) apoptosis of HeLa cells treated with G1 and M/CdS-HA+L, G2 (LA (10mM)), G3 (5mm chicken breast), G4 (ammonium chloride solution) and G5 (hypoxia) (P compared with G1).

Figure 6.
(a) enhanced diffusion by temperature rise (T), MCSs formulation and penetration mechanism with reduced TIP, MXene+L, M/CdS, M/CdS+L and Mash CDS Lateral Genome; (b) laser irradiation on the fluorescence image of MCSs processed with M/CdS/HA/RB on days 0 to 6 (scale bar = 100 ¦Ì m). (C) MCS images processed with MXene+L, M/CDS, M/CDSAD+L and M/CDS+L+Gen on days 0 and 6 (scale bar = 100 ¦Ì m). (d) Cell viability of MCSs treated with MXene+L, M/CdS, M / CDs + L and M/CdS+L+Gen on day 0 and day 6.
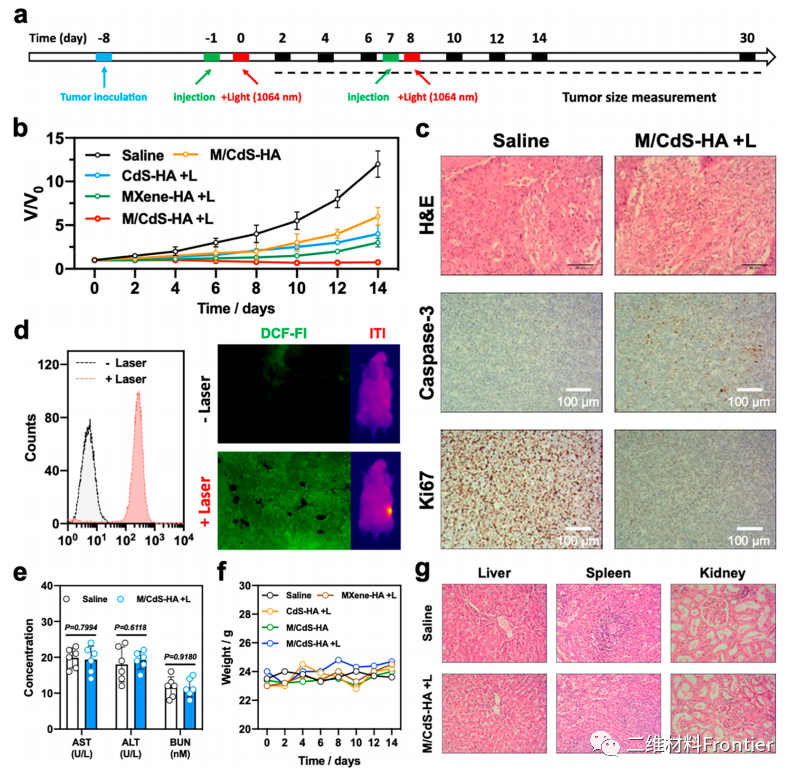
Figure 7.
(a) Experimental description of anti-tumor therapy in vivo. (B) tumor growth curves of all tested groups. (C) Hype staining / enzyme-3-Ki67), M/CdS-HA+L and photothermal effect of H/CdS-HA+L detected by saline, FCM, DCF-FI and HTI. (e) Serum biochemical data of AST, ALT and BUN. (F) weight changes of all tested groups. (G) histological staining of the main organs (liver, spleen and kidney) of mice treated with saline and M/CdS-HA+L (scale strip = 50 ¦Ì m).
III. Summary of the full text.
Although nano-amines have brought great hope for tumor therapy in recent years, their application is seriously limited by the low efficiency of penetrating tumors. Previous studies have focused on drug size reduction, tumor microenvironment remodeling, and cross-cell division to enhance tumor penetration. However, none of these strategies overcomes the key obstacle to drug delivery: TIP, especially TIFP. Therefore, water splitting based on pyroelectric catalysis is used to reduce TIFP and high heat to reduce TISP. Because this work simulates lymphatic function to enhance blood perfusion, it is identified as nanolymph. In addition, hyperthermia and ROS produced by NIR-II laser irradiation can effectively damage tumor cells. As a benefit of reduced TIP, nanolymph exhibits enhanced tumor penetration and inhibition of tumor proliferation and recurrence. It is proved that lymphatic nanotubes have superior anti-tumor effect in vitro and in vivo. Before studying drug permeability, the relationship between water content in tumor and TIP was revealed, and TIP could be reduced by water division in tumor. In the study of the drug, the enhancement of nano-lymphatic penetration was demonstrated by the use of penetration, simulated tumor ECM, three-dimensional tumor spheres and solid tumors in mice. The combination of hyperthermia and tumor interstitial fluid decomposition can significantly reduce TIP. Among them, TIFP is the main component of TIP. In addition, nanolymph can reduce the number of LA in tumor, which indicates that LA is a sacrificial agent that reacts with positive charge and inhibits the recombination of load carriers to enhance the formation of ROS. Based on the above results, the authors identified the tumor penetration therapy strategy based on water division and ROS generation as hydrodynamic therapy.
This information is from the Internet for academic exchange only. if there is any infringement, please contact us to delete it immediately.










