What is MXene?
QQ Academic Group: 1092348845
Detailed
MXenes, first discovered in 2011, are ceramics that comprise one of the largest families of two-dimensional (2D) materials.
MXenes are made from a bulk crystal called MAX. 2D layered materials derived from MAX or non-MAX phases were not predicted to exist before this discovery. Unlike most 2D ceramics, MXenes have inherently good conductivity and excellent volumetric capacitance because they are molecular sheets made from the carbides and nitrides of transition metals like titanium. MXenes have already found applications ranging from energy storage to medicine and optoelectronics.
What makes MXenes so interesting is the fact that this material class could conceivably consist of any of millions of possible arrangements of transition metals (like molybdenum or titanium), carbon and nitrogen. The trick is to find the ones that are stable.
By using a high-throughput computational platform and scanning through the formation energies of millions of alloying configurations, researchers estimate that there are more than a million of stable MXene compounds remaining to be discovered (ACS Nano, "High-Throughput Survey of Ordering Configurations in MXene Alloys Across Compositions and Temperatures").
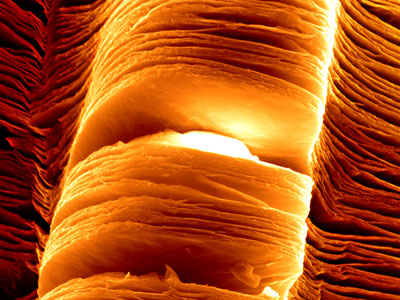
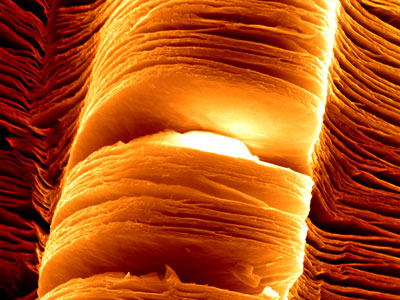
Scanning electron micrograph of exfoliated MXene nanosheets. (Image: Babak Anasori, Drexel University)
MAX phases
There is a large family of ternary carbides (ternary is an adjective meaning ‘composed of three‘) with the general formula Mn+1AXn, where n = 1–3, M denotes a transition metal, A is an element such as aluminum or silicon, and X is either carbon or nitrogen. Researchers have termed these ductile and machineable ceramics MAX phases.
As a consequence of their layered structure, these materials kink and delaminate during deformation and also exhibit an unusual, and sometimes unique, combination of properties; they are not sure whether they want to be metals or ceramics. While they conduct heat and electricity like metals, they are elastically stiff, strong, brittle, and heat-tolerant like ceramics. They are resistant to chemical attack, readily machinable, and thermal shock, damage tolerant, and sometimes fatigue, creep, and oxidation resistant.
MXene discovery
Two-dimensional (2D) structures, like graphene and molybdenum disulfide, are known to have unique properties. Therefore, having a new family of 2D structures with a wide range of chemistries can open the door for better understanding of differences between properties of 2D and 3D materials, lead to identification of useful properties of 2D carbides, nitrides, oxycarbides and other related structures, and finally result in new applications.
MAX Phases have been researched for years and dozens of layered carbides, nitrides and carbonitrides with a variety of properties have been synthesized.
have been researched for years and dozens of layered carbides, nitrides and carbonitrides with a variety of properties have been synthesized.
However, these ceramics have always been produced as three-dimensional materials, until researchers placed titanium-aluminum carbide (Ti3AlC2) powders in hydrofluoric acid at room temperature to selectively remove the aluminum. The result of this chemical process – referred to as exfoliation – essentially spreads out the layered carbide material and yields two-dimensional Ti3C2nanosheets, which have since been coined MXene, as a kin to graphene.
In a 2011 paper in Advanced Materials ("Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2"), researchers first demonstrated this ability to transform three-dimensional titanium-aluminum carbide, a typical representative of MAX phases, into a two dimensional structure with greatly different properties.
How to make MXene
MXenes are created by selectively removing aluminum from layered MAX phases. Through this exfoliation process, the carbide layers are separated into two MXene sheets just a few atoms thick. MXenes can accommodate various ions and molecules between their layers by a process known as intercalation, which is sometimes a necessary step in order to exploit the materials’ unique properties.
For example, placing lithium ions between MXene sheets has been shown to render them promising materials for both lithium-ion batteries and electrochemical capacitors.
To synthesize free-standing MXene flakes, a research team from Drexel University improved their initial technique from 2011 using acid, which they termed minimally intensive layer delamination (MILD). They treated bulk MAX with an etchant of fluoride salt and hydrochloric acid to selectively remove unwanted layers of aluminum from between titanium carbide layers.
Then they manually shook the etched material to separate and collect the titanium carbide layers. Each layer is five atoms thick and is made of carbon atoms binding three titanium sheets. Etching and exfoliating MAX produces many of these free-standing MXene layers. This relatively simple technique may enable manufacturing-scale production.
Since then, the continued exploration has revealed their exceptional ability to store energy, block electromagnetic interference, purify water and even ward off bacteria. And, as recent research suggests, MXenes are also very durable – the strongest material of its kind.
Although there are many possible MXene alloy compositions, most will not be stable. The challenge faced by material scientists has been how to efficiently sweep through the huge number of alloy configurations to identify those with the lowest formation energy and hence highest stability. Conventional ‘first principles’ calculation approaches are too computationally intensive for such a scan to be feasible.
A high-throughput scan of possible compositions for MXenes gives researchers invaluable direction for picking the best candidate from the millions of possible material recipes.
MXene uses and applications
MXene could be used in energy storage devices such as electrodes of Li-ion batteries, pseudo capacitors, etc. The researchers also envision its use as reinforcement in composites, similar to clays or graphene, which increase mechanical properties and decrease gas permeability of polymers. A variety of surface chemistries, presence of transition metal oxides and high surface area make MXene potentially attractive for catalytic applications.
Desalination and waste water treatment
The materials‘ remarkable properties open new possibilities for MXenes in water desalination and wastewater treatment. This excitement comes from findings that Ti3C2 can trap the energy of sunlight to purify water by evaporation with an energy efficiency that is state-of-the-art.
To investigate MXene’s possibilities in water purification, researchers fabricated a thin and flexible Ti3C2 membrane incorporating a polystyrene heat barrier to prevent the heat energy from escaping. This created a system that could float on water and evaporate some of the water with 84% efficiency at the illumination levels of natural sunlight.
Battery technology and energy storage
Computational studies have suggested that fully exfoliating, or delaminating, certain MXenes would yield layers with exceptional charge capacities for use in battery anodes. In one report, scientists demonstrated successful intercalation of MXenes with several organic molecules, including dimethyl sulfoxide (DMSO), which allowed them to fully exfoliate stacked layers into MXene sheets and ultimately create MXene ‘paper‘ by filtering flakes from solution.
This flexible and electrically conductive paper showed a lithium ion capacity of four times that of typical MXene material, with extremely high charging rates and a cyclability superior to graphite, which is used in commercial lithium-ion batteries. Critically, this work demonstrates that such material can be synthesized on a large scale.
Researchers also have developed new electrode designs with MXene material, that will allow batteries to charge much faster. Their design could make energy storage devices like batteries, viewed as the plodding tanker truck of energy storage technology, just as fast as the speedy supercapacitors that are used to provide energy in a pinch – often as a battery back-up or to provide quick bursts of energy for things like camera flashes.
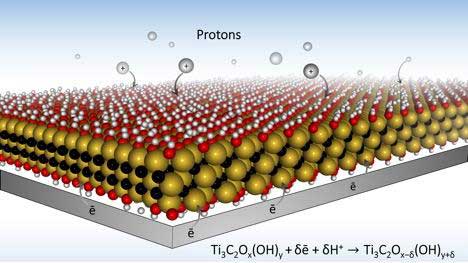
New electrode designs using MXene material will allow batteries to charge much faster. The key is a microporous design that allows ions to quickly make their way to redox active sites. (Image: Drexel University)
Triboelectric nanogenerators
Researchers have demonstrated that MXenes could be used to harvest wasted frictional energy, for example, from muscle contractions during typing or walking. MXenes possess high electrical conductivity and the ability to uptake electrons when in contact with polymers and other materials.
This unusual combination of properties makes them useful as components for triboelectric nanogenerators (TENG), which turn muscle movements into electric power. The research suggests these advanced materials could be incorporated into mobile phones, handheld electronics, wearable devices and laptops, ultimately making them self-powering.
Conductive coatings
MXenes have been used to developed a mechanically robust conductive coating that can maintain performance under heavy stretching and bending. This research exploited the fact that MXene multilayer coatings that can undergo large-scale mechanical deformation while maintaining a high level of conductivity. In this work, the researchers also successfully deposited the MXene multilayer coatings onto flexible polymer sheet, stretchable silicones, nylon fiber, glass and silicon.
Sensors and chemical noses
It appears that MXene is one of the most sensitive gas sensors ever reported. This research is significant because it expands the range for detection of common gases allowing us to detect very low concentrations that we were not able to detect before.
Research findings suggest that MXene can pick up chemicals, such as ammonia and acetone, which are indicators of ulcers and diabetes, in much lower traces than sensors currently being used in medical diagnostics.
MXene‘s advantage over conventional sensor materials lies in its porous structure and chemical composition. The material is good at both allowing gas molecules to move across its surface and snagging, or adsorbing, certain ones that are chemically attracted to it, showing good selectivity.
- Previous: Switching the peroxida
- Next: What is graphene?


 Application
Application