[Transfer] Mesoporous covalent organic framework thin layer is used for high charge and discharge rate electric double layer capacitor!
QQ Academic Group: 1092348845
Detailed
In the field of electrochemical energy storage, electric double layer capacitors (EDLCs) achieve charge storage through the rapid adsorption of electrolyte ions on the electrode-electrolyte interface, which has higher power density and cyclic stability compared to pseudocapacitors and batteries Sex. Although EDLC is considered as a promising energy storage device, currently only microcapacitors based on graphite carbon electrodes can store and transfer charge at a relatively high charge and discharge rate (greater than 1000 mV s-1). The only way to build a microcapacitor architecture depends on complex and expensive laser writing and / or lithography techniques. Therefore, the development of double-layer capacitors with low cost, high charge and discharge rate, and non-graphite carbon electrode material is of great significance.
COFs can accurately design the periodic skeleton and unique pore structure, showing its great potential in energy storage. Several 2D COFs in energy storage applications that have been reported so far are only used as electrode active materials for pseudocapacitors. Professor Fang Qianrong of the State Key Laboratory of Inorganic Synthesis and Preparative Chemistry at Jilin University and his team used mesoporous covalent organic framework thin layers (e -COFs) as the electrode material for the construction of capacitors, for the first time, the application of COFs in electric double layer capacitors was achieved, and good double-layer charge storage performance was obtained.
[Design and synthesis of e-COFs]
1. Preparation of granular COFs by solvothermal method. 6M acetic acid as the catalyst, anhydrous 1,2-dichlorobenzene and anhydrous ethanol as the solvent, heated at 130 ℃ for 4 days, obtained COFs (JUC-510, JUC-511 and JUC-512). 2. Liquid-phase stripping to prepare thin-layer e-COFs . These COFs were stripped by a simple and gentle chemical stripping technique, that is, each COFs was dispersed in isopropyl alcohol and sonicated for 7 h, and then a uniform colloidal mixture was obtained. The powder separated by filtration and drying is expressed as e-COF (e-JUC-510, e-JUC-511 and e-JUC-512).
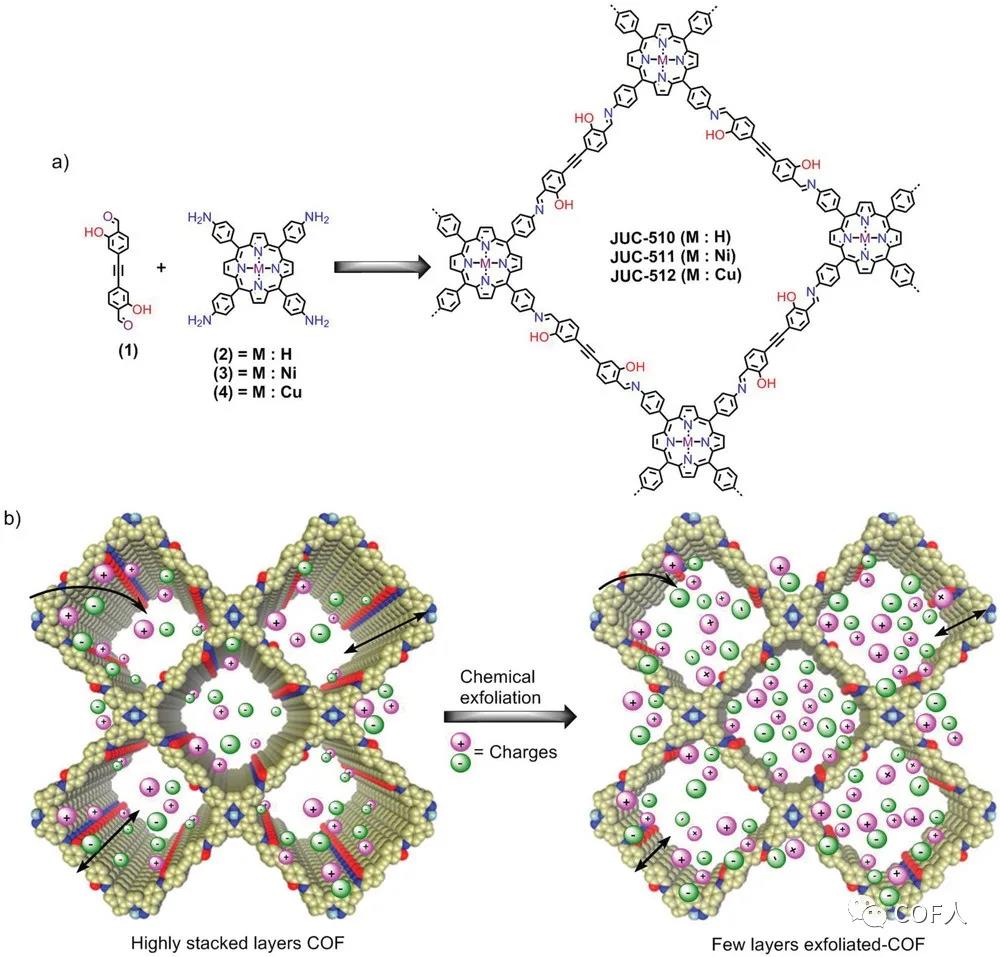
Figure 1 COFs series design synthesis and COFs and e-COFs charge storage diagram
[Structure]COFs (JUC-510, JUC-511, and JUC-512) formed by porphyrin-based building blocks have overlapping AA layer stacking modes, square open channels with a diameter of 3.4 nm, and good BET surface area (up to 1170 m2 g-1), high thermal and chemical stability , is an equiaxed crystal with a highly aggregated rod-like morphology. Since there is no metal on the porphyrin group of JUC-510, the specific surface area is slightly higher than JUC-511 and JUC-512.
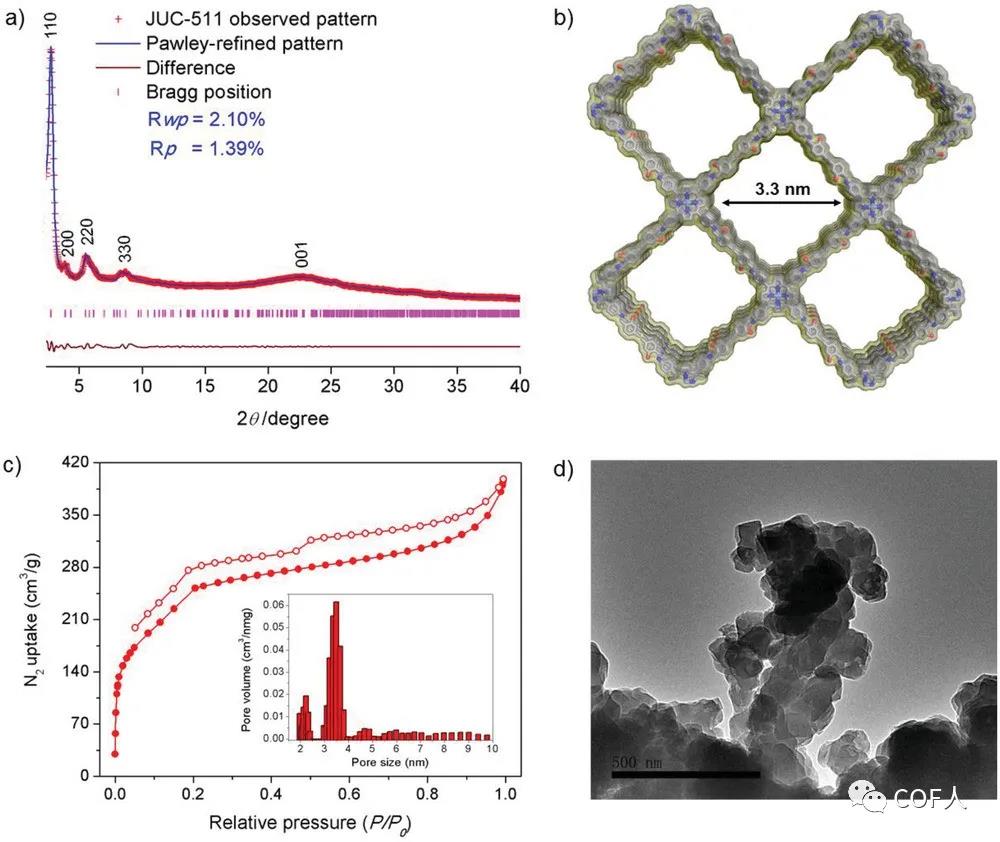
Figure 2 JUC-511 structure and porosity analysis
After peeling these COFs by a simple and gentle chemical peeling method , thin layer structures (e-COFs) with an average thickness of about 22 nm were obtained, although the specific surface area was significantly reduced (e-JUC-510 was the highest, 666 m2 g-1 ), But still much higher than other reported covalent organic nanosheets, and the peeled e-COFs structure is not deformed, showing a tenon layered morphology, thermal stability is maintained, and still has a good layered structure and The larger aperture is a promising EDLC electrode material.
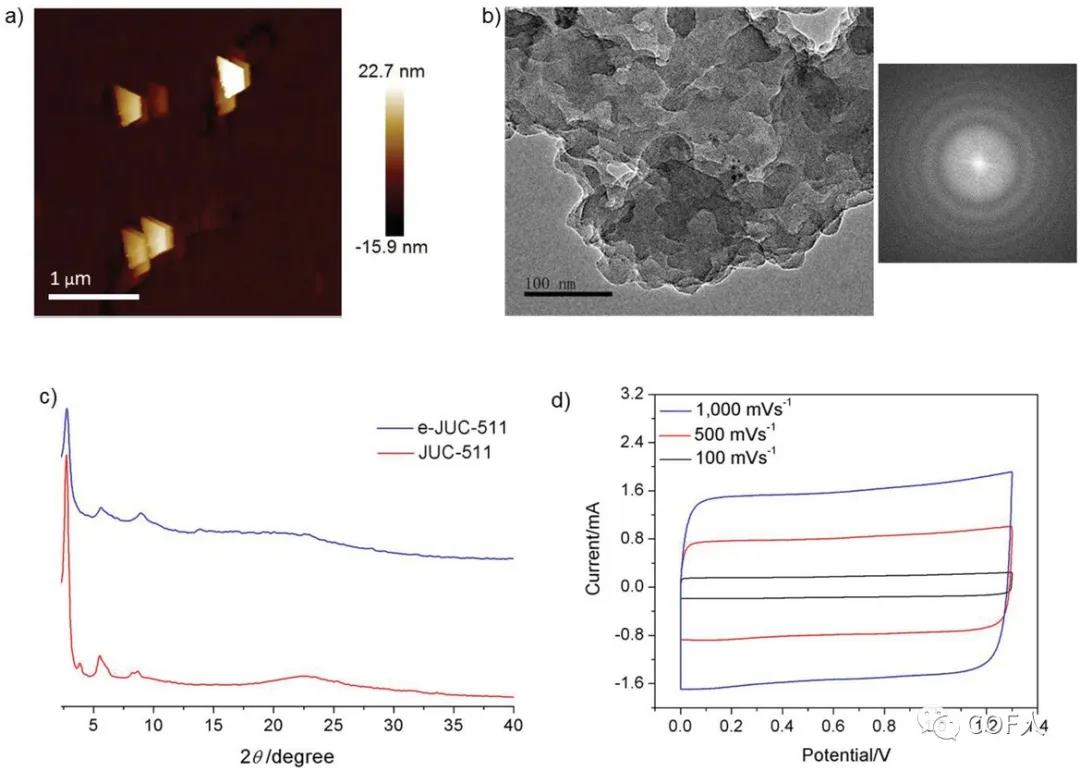
Figure 3 Microstructure and electrochemical analysis of e-JUC-511
[Performance]Using COFs or e-COFs (60%), conductive carbon black (30%) and a few drops of PTFE (10%) as binders, electrodes composed of COFs and e-COFs were prepared, using aluminum foil as the current collector Finally, the aluminum foil was cut into a circular electrode with a diameter of 1.2 cm (mass load 0.678 mg / cm2, thickness 10 m). Furthermore, two identical circular electrodes (as negative electrode and positive electrode) were taken, separated by an ion porous membrane (Celgard 3501), and an organic electrolyte of 1M NBu4PF4 / ACN was used as the electrolyte to prepare a brand new non-graphite carbon electrode capacitor. It is worth noting that even at 1000 mV s -1 or higher, the CV curve of the e-COF capacitor battery still shows an almost ideal rectangular curve, and no obvious redox peaks are found, indicating that double electric Layer charge storage . Since e-JUC-511 and e-JUC-512 both contain electroactive metals (Ni and Cu), the ionic conductivity of the corresponding e-COF can be improved, so the battery rate performance of e-JUC-510 capacitors is slightly lower, but with Traditional graphite carbon capacitors are still not to be underestimated. The results showed that, e-COF-511 double-layer capacitor having a high area ratio of the capacitance (5.46 mF cm-2) and quality power (55 kW kg-1), a relatively low value τ0 (MS 121) , and may be a high charge At the discharge rate (up to 30000 mV s-1), the charge is transferred, and at the same time, it can maintain nearly 100% of the capacitance after 10,000 cycles . CV, EIS and GCD analysis confirmed that the stripping method shortens the entire active surface ion transport path of the electrode material and provides rapid ion mobility, achieving a high charge discharge rate and excellent capacitance performance of the e-COF capacitor. Finally, it is assembled in series or parallel configuration to achieve a certain working voltage or output current. The working voltage window and capacity show good control, which once again proves the stability and reliability of the e-COF capacitor battery.

Figure 4 Capacitance performance of e-COF capacitor
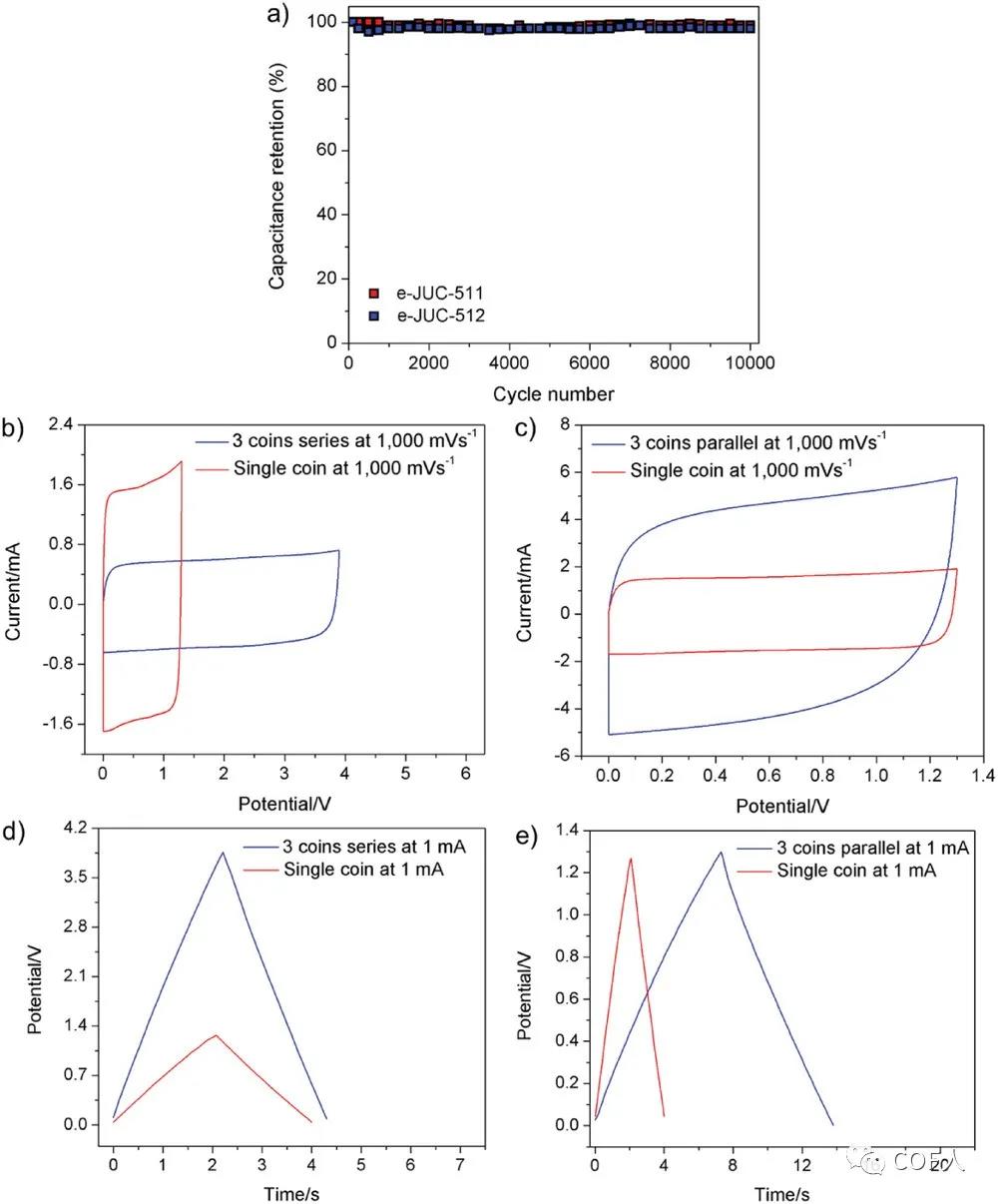
Figure 5 Stability and reliability analysis of e-COF capacitors
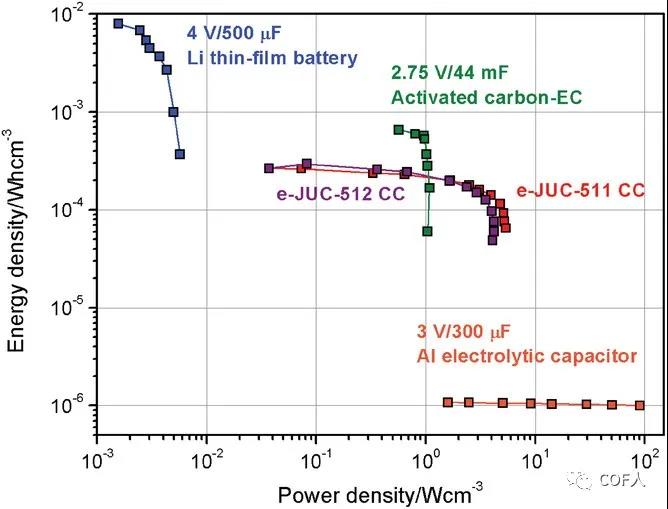
Figure 6 Ragone diagram of e-COF capacitor
[Summary]This article uses ultrasonic peeling technology to prepare 2D mesoporous COFs and successfully assembles electric double layer capacitors, with excellent surface capacitance (5.46 mF cm 2 at 1000 mV s -1) and mass power (55 kW kg -1), showing the potential application prospects of COFs materials in electric double layer capacitors, providing new ideas for the potential applications of COFs in energy storage.
Original link: https://doi.org/10.1002/adma.201907289
Source of information: MOFs online
This information originates from the Internet for academic exchange only. If there is any infringement, please contact us to delete it immediately
- Previous: Metal-free thiophene-s
- Next: A Rising 2D Star: Nove


 Academic Frontier
Academic Frontier
