Where is the recognition of MXene, numbers speak!
QQ Academic Group: 1092348845
Detailed
Introduction to MAX Phase and MXenes
The general formula of the composition of the MAX phase is Mn + 1AXn, where M is a transition metal element, as shown in the light green part of FIG. 1, A is mainly the element of the third or fourth main group, as shown in the red part of FIG. 1, X is carbon and nitrogen Or a combination of the two, n can take the values of 1, 2, 3, 4 and are called MAX phases of type 211, type 312, type 413, type 514, respectively. Under the premise of stoichiometric ratio, the M elements can be combined with each other, namely Bimetal or multimetal, Ti2AlC and (V0.5Cr0.5) 2AlC are 211 type, Ti3AlC2 and Ti3SiC2 are 312 type, Ti4SiC3 and Ti4AlN3 are 413 type. When n = 1, the type 211 is composed of one regular octahedron, when n = 2, the type 312 is composed of two regular octahedrons, when n = 3, the type 413 is composed of three regular octahedrons As shown in Figure 2, it is a schematic diagram of the structure of several MAX phases and corresponding MXenes.
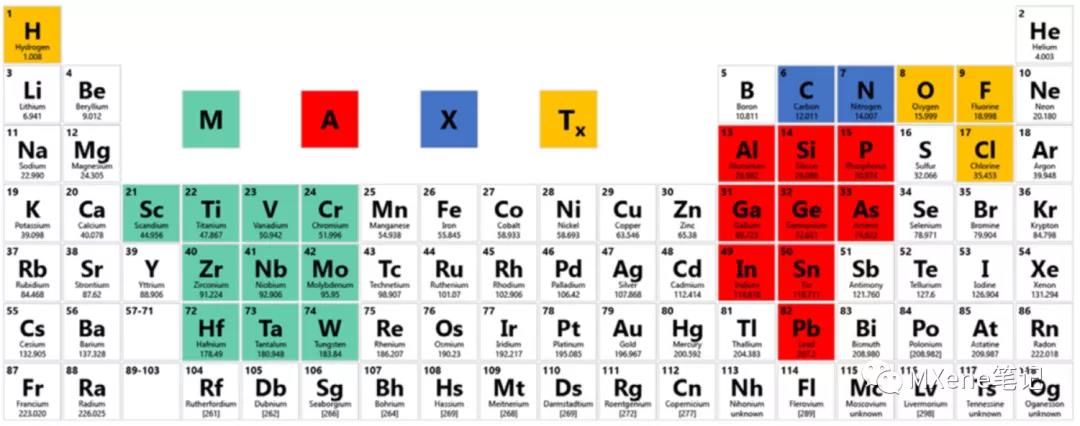
Figure 1 The three elements in MAX that have been reported, light green is M, red is A, and blue is X.
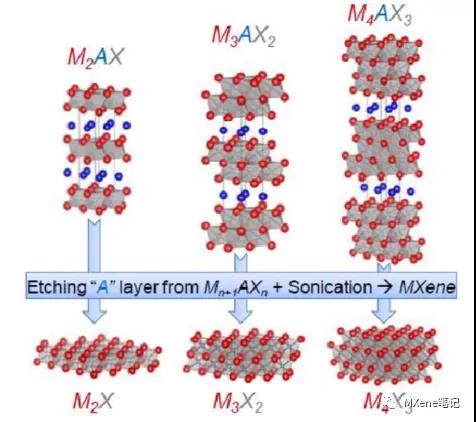
Figure 2 Schematic diagram of the structure of MAX phase and corresponding MXenes
It has been reported that there are more than 150 kinds of MAX. By selectively removing the A element (usually HF acid etching), a new type of two-dimensional material MXenes can be obtained. There are more than 30 kinds of MXenes synthesized. There are many types, which provide a large number of precursors for the synthesis of MXenes. [1]
MXenes advantages
①MXenes has excellent conductivity (conductivity 10 ^ 5 S / m), and the conductivity reaches some metal levels.
Table 1 MXenes and other materials conductivity comparison
|
material |
Electrical conductivity ( S / m ) |
Remarks |
|
silver |
6.3X10 ^ 7 |
The most conductive metal |
|
copper |
5.9X10 ^ 7 |
Common wire |
|
aluminum |
3.7X10 ^ 7 |
Common wire |
|
magnesium |
2.2X10 ^ 7 |
|
|
lithium |
1.3X10 ^ 7 |
|
|
tin |
8.7X10 ^ 6 |
|
|
titanium |
2.3X10 ^ 6 |
|
|
cerium |
1.2X10 ^ 6 |
|
|
HG |
10 ^ 6 |
|
|
bismuth |
9.5X10 ^ 5 |
On the same order of magnitude as M X enes , conductivity reaches metal level
|
|
gadolinium |
7.3X10 ^ 5 |
|
|
M X enes |
2.4X10 ^ 5 |
Single layer / few layers M X enes , suction filtration into thin film test |
|
graphite |
7.7X10 ^ 4 ~ 1.2X10 ^ 5 |
High quality M X enes conductivity is slightly better than graphite |
|
Graphene |
10 ^ 8 |
|
|
silicon |
2.5X10 ^ -4 |
semiconductor |
Recently, some literature refreshed the conductivity of MXene, reaching 15100S / cm, which is an order of magnitude higher than graphite, but inferior to graphene.
②Good hydrophilicity (carbon nanotubes and graphene have a stable carbon surface, the hydrophilic property is very limited), this advantage provides convenience for the composite of MXenes and other materials. [2]
③MXenes has good mechanical properties (better tensile strength and flexibility), and it is easy to form a film and directly used as an electrode. When used as a battery pole piece, it avoids the addition of binders and conductive agents. [3]
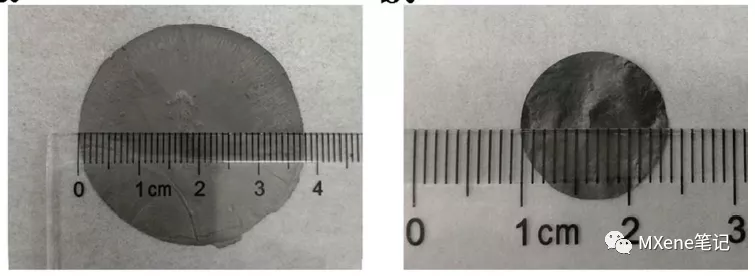
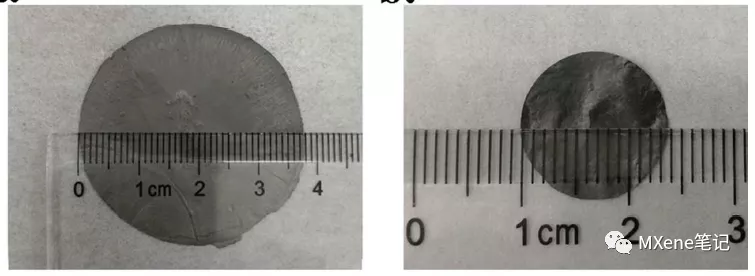
Figure 3 The physical picture of MXenes membrane obtained by suction filtration
④ The layer spacing is large and the flexibility is adjustable. The MXenes layer spacing obtained by etching ultrasound is about 1nm (based on the 312 type MAX phase etching). The van der Waals force is between the layers. The layer spacing is flexible and does not damage its layers. Structure (the layer spacing can be pillared to 2.5nm). In view of this advantage, MXenes-based composite materials are prepared by compounding and doping. By introducing a pillaring agent between the MXene layers, due to the pillaring effect (using ion impact or with the layer Interaction of related chemical bonds, or interlayer fillers, effectively expand the MXenes layer spacing, so that the MXenes layer has a larger storage load space), the MXenes layer spacing will increase, which can provide greater storage and load ion Transport space to promote electrolyte wetting. Due to the limiting effect between the layers, the active material material filled between the MXene layers can be prevented from breaking and pulverizing. In addition to providing its own capacity, the volume expansion during the charging and discharging process can further increase the MXene layer spacing, so that More ions (Li +, Na +, etc.) are embedded between MXene layers, giving MXene a higher capacity. [4-5] In addition, the MXenes layer spacing increases with the increase of n value, such as the 413 type MAX phase etching MXenes layer spacing is 1.4nm.
⑤O, OH, F and other functional groups on the surface of the stripped MXenes are electronegative, such as Ti3AlC2 after etching to remove the Al element should be Ti3C2, but because the surface adsorbs three main functional groups of O, OH, F, often write Ti3C2Tx (Tx represents the functional group adsorbed on the surface). The functional groups on the surface of MXenes have the function of adsorbing polysulfides (the adsorption of sulfur by carbon materials, because they are non-polar, usually physical adsorption, and MXenes have physical and chemical double adsorption), thereby inhibiting the shuttle effect of polysulfides in lithium-sulfur batteries To improve the electrochemical performance of lithium-sulfur batteries. When MXenes are used as positive sulfur carriers for lithium-sulfur batteries, separator interlayers, and for improving lithium metal negative electrodes, battery performance is significantly improved. [6]
Table 2 Common material layer spacing
|
material |
Interlayer distance ( nm ) |
|
graphite |
0.33 |
|
Black phosphorus |
0.53 |
|
Molybdenum disulfide |
0.65 |
|
M X enes (312 M AX etching ) |
1 |
MXenes disadvantages
① The peeled MXenes flakes are easy to stack and reunite, which makes the Mxene flakes dense and it is difficult to prepare simple MXenes powder with few layers. After the MXenes sheet is agglomerated and densified, it makes it difficult for the electrolyte to infiltrate, and it cannot exert its electrochemical performance. In view of this shortcoming, the target application of MXenes (MXenes has good hydrophilicity) is often started when a small amount of MXenes aqueous solution is obtained. [7]
②When MXenes alone is used as the electrode negative electrode material, the first Coulomb efficiency is low (58%), and the specific capacity is low (used as a lithium battery negative electrode, the theoretical capacity is 320mAh / g, which is lower than graphite‘s 372 mAh / g). The platform is widely used as a capacitor material for research, usually doping and recombination are needed to improve. [8]
Main applications of MXenes energy storage
①Use the layered structure to play the role of conductive skeleton
②Topography design (hollow ball, flower, porous)
③ Composite flexible self-supporting film, eliminating the need for adhesives (generally available by direct suction filtration)
④Combination with high-capacity materials (MXenes exerts electrical conductivity and suppresses volume expansion of other active materials)
MXenes recognition
① Large layer spacing and flexible adjustment
② Rich surface chemistry (that is, the surface of the obtained MXenes contains functional groups, which makes MXenes have the characteristics of hydrophilicity, surface electronegativity, etc.).
Although the conductivity is good, the conductivity is no better than graphene, and even some MXenes are semi-conducting. Although the specific surface area is large, it is tens to hundreds of square meters per gram, which is no better than MOF materials (thousands of square meters per gram). Good electrical conductivity and large specific surface area are characteristics of MXenes, but they are not unique to MXenes.
[1] Alhabeb M, Maleski K, Anasori B, et al. Guidelines for Synthesis and Processing of 2D Titanium Carbide (Ti3C2Tx MXene) [J]. Chemistry of Materials, 2017, 29, 7633−7644
[2] Ling Z, Ren CE, Zhao MQ, et al. Flexible and conductive MXene films and nanocomposites with high capacitance [J]. Proceedings of the National Academy of Sciences, 2014, 111 (47): 16676-16681.
[3] Zhao M Q, Ren CE, Ling Z, et al. Flexible MXene / Carbon Nanotube Composite Paper with High Volumetric Capacitance [J]. Advanced Materials, 2015, 27 (2): 339-345.
[4] Luo J, Zhang W, Yuan H, et al. Pillared Structure Design of MXene with Ultra-Large Interlayer Spacing for High Performance Lithium-Ion Capacitors [J]. Acs Nano, 2017, 11: 2459-2469.
[5] Luo J, Fang C, Jin C, et al. Tunable Pseudocapacitance Storage of MXene by Cation Pillaring for High-Performance Sodium Ion Capacitors [J]. Journal of Materials Chemistry A, 2018, 6, 7794-7806.
[6] Tang X, Guo X, Wu W, et al. 2D Metal Carbides and Nitrides (MXenes) as High-Performance Electrode Materials for Lithium-Based Batteries [J]. Advanced Energy Materials, 2018.
[7] Hollow MXene Spheres and 3D Macroporous MXene Frameworks for Na‐Ion Storage [J]. Advanced Materials, 2017, 29 (37).
[8] Pang J, Mendes RG, Bachmatiuk A, et al. Applications of 2D MXenes in energy conversion and storage systems [J]. Chemical Society Reviews, 2018.
Source:
- Previous: Two-dimensional (2D) m
- Next: What is graphene?


 Application
Application