
hotline:
17715390137
Tel/Wechat:
18101240246 (Technology)
0512-68565571
Email:mxenes@163.com (Sales Engineer)bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online

introduction
Zinc-air batteries have become one of the most promising metal-air battery technologies due to their high energy density and safety. On the one hand, zinc is rich, has lower cost and higher renewable potential compared to lithium. On the other hand: the Zn metal negative electrode has a relatively high redox potential, which enables it to operate in an aqueous electrolyte. Zinc anodes can achieve reversible Zn deposition / stripping under conditions close to neutral or weakly acidic electrolytes (pH 3.6–6.0), and have a long cycle life. In addition, the Zn electrochemical reaction involves two electrons, resulting in a higher volume energy density. However, the large-scale application of zinc-air batteries is limited by the slow kinetics of oxygen reduction reaction (ORR) and oxygen release reaction (OER) in air electrodes. Therefore, it is necessary to develop a dual-function electrocatalyst having high performance. Platinum (Pt) has been regarded as the benchmark for ORR electrocatalysts and has been widely used in renewable energy equipment. However, its high cost, low OER activity, and its poor durability prevent it from being used alone for dual-function electrocatalysts. Combining Pt with another low cost material with high OER activity is considered an attractive strategy to achieve high performance dual function electrocatalysts.
Achievements
Recently, Prof. Shao Zongping and Prof. Zhou Yi (co-corresponding author) of Nanjing University of Technology have synthesized Fe-doped SrCo0.95P0.05O3−δ perovskite analogs by solid-phase reaction route, which is denoted as Sr (Co0.8Fe0.2 ) 0.95P0.05O3-δ (SCFP). SCFP was then uniformly mixed with 20 wt% of Pt / C (20Pt / C) commercial material and Super P Li additive using a simple ball milling method. The obtained composite material is represented as Pt-SCFP / C-ab, and ab represents a mass ratio of Pt (a) to SCFP (b). Pt-SCFP / C-12 shows excellent bifunctional catalytic activity, its OER performance is better than IrO2 catalyst, and its ORR performance is close to that of Pt / C samples. The Zn-air battery based on the Pt-SCFP / C-12 catalyst has excellent cycle stability. At the same time, the battery has a high peak power density of 122 mW cm-2 at 214 mA cm-2 and a high specific capacity of 790.4 mAh g-1 at a current density of 5 mA cm-2. The excellent electrochemical performance can be attributed to the rapid transfer of electrons in Pt-O-Co, the synergistic effect between Pt and SCFP, and the large number of oxygen vacancies. This work not only provides a series of low-cost, highly active electrochemical catalysts for zinc-air batteries, but also provides a simple and effective method for the practical application of zinc-air batteries. The related research achievement "High-Performance Platinum-Perovskite Composite Bifunctional Oxygen Electrocatalyst for Rechargeable Zn-Air Battery" was published on Advanced Energy Materials.
Graphic guide
Figure 1.Phase characterization and composition characterization of the catalyst
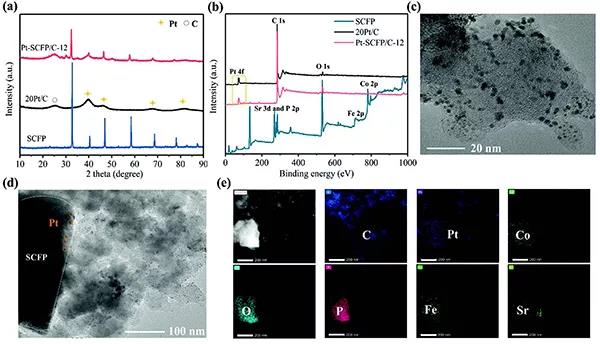
(A) XRD spectrum and XPS spectrum of SCFP, 20Pt / C and Pt-SCFP / C-12 composite catalyst.
(C-e) HRTEM image of Pt-SCFP / C-12 and corresponding element mapping.
Figure 2.Characteristics of the electrocatalytic performance of the catalyst
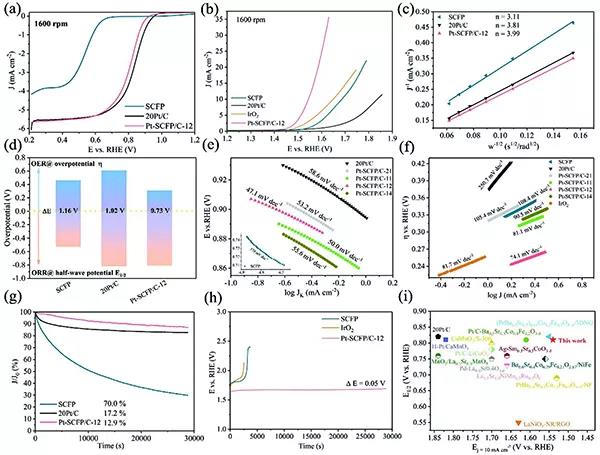
(A, b) LSV curves for SCFP, 20Pt / C and Pt-SCFP / C-12 ORR and OER tests.
(C) ORR K-L diagrams for SCFP, 20Pt / C and Pt-SCFP / C-12.
(D) Comparison of potential differences between SCFP, 20Pt / C and Pt-SCFP / C-12.
(E, f) ORF of SCFP, 20Pt / C and Pt-SCFP / C-ab composites, and Tafel diagrams of SCFP, 20Pt / C, IrO2 and Pt-SCFP / C-ab.
(G, h) Comparison of catalyst stability.
(I) Comparison with other work.
Figure 3.Characteristics of electrochemical performance of zinc empty battery based on Pt-SCFP / C-12 catalyst
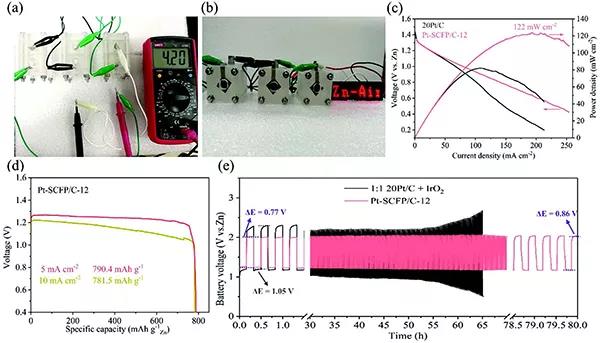
(A) Open circuit voltage of three Pt-SCFP / C-12 based Zn-air batteries in series
(B) The zinc-air battery based on Pt-SCFP / C-12 powers the LED screen.
(C) Polarization diagram and corresponding power density curve of zinc-air battery.
(D) Constant current discharge curve.
(E) Voltage-time curve of Zn-air battery based on Pt-SCFP / C-12 or 1: 1 20Pt / C + IrO2 discharge at 5 mA cm-2.
Figure 4.Mechanical characterization of the catalyst
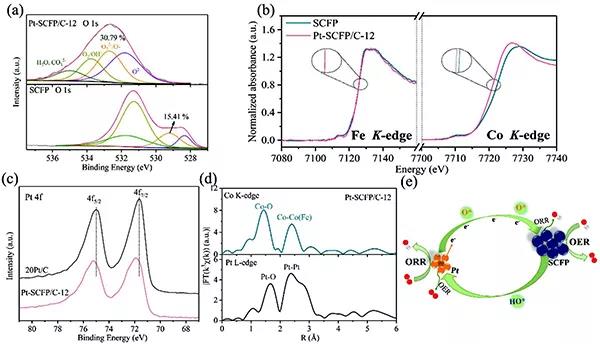
(A) O 1s XPS spectra of Pt-SCFP / C-12 and SCFP.
(B) XANES Co and Fe K edge spectra of SCFP and Pt-SCFP / C-12.
(C) Pt 4f XPS spectra of 20Pt / C and Pt-SCFP / C-12.
(D) Pt-SCFP / C-12 Rt spectrum of Pt L-edge and Co K-edge EXAFS.
(E) Schematic diagram of electronic interactions and spillover effects in Pt-SCFP / C-ab composites for ORR and OER.
Figure 5 First-principles calculations
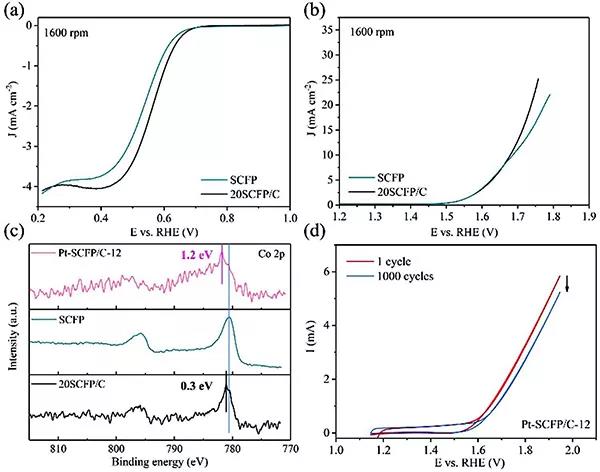
(A) LSV curve of OER and ORR in alkaline solution
(C) Co 2p XPS spectra of Pt-SCFP / C-12, SCFP and 20SCFP / C samples.
(D) Pt-SCFP / C-12 catalyst was subjected to 1 cycle and 1000 cycle CV curves in 0.1 m KOH ADT.
summary
In summary, in this work, a series of Pt-SCFP / C-ab composites based on perovskite oxide SCFP and precious metal 20Pt / C were synthesized by a simple ball milling method. Then, the coexistence of Pt and SCFP phases in the Pt-SCFP / C-12 complex and its uniform distribution on the carbon surface were determined by XRD, STEM and element mapping. Pt-SCFP / C-ab composites show excellent ORR and OER performance. The best Pt-SCFP / C-12 sample has excellent dual-function catalytic performance and durability, which is superior to the OER performance of IrO2 catalyst. 20 Pt / C sample. The excellent electrochemical activity can be attributed to the rapid electron transfer through the Pt-O-Co bond, the decrease of the energy barrier and the increase of active sites and surface oxygen vacancies. In addition, the battery equipped with Pt-SCFP / C-12 also has excellent charge and discharge cycle stability, which can maintain 80 hours (240 cycles). This kind of precious metal catalyst can be used to reduce the application cost and improve the electrochemical performance at the same time, and its economic application potential in metal-air batteries and other devices is very huge.
Literature link: "High-Performance Platinum-Perovskite Composite Bifunctional Oxygen Electrocatalyst for Rechargeable Zn–Air Battery" (Adv. Energy Mater. 2019.DOI: 10.1002 / aenm.201903271)
Corresponding author
Zhou Yi, Professor, PhD Supervisor, State Key Laboratory of Materials Chemical Engineering, School of Chemical Engineering, Nanjing University of Technology. Young scholars of overseas high-level talent introduction plan, winners of Jiangsu Outstanding Youth Fund, Jiangsu Distinguished Professor, Jiangsu Province “Double Creation Talents”, Jiangsu Province “Double Creation Teams” Leading Talents. He has published more than 250 SCI papers in international journals such as Nature Energy, Nature Communications, Science Advances, Advanced Materials, etc .; the paper has been cited more than 9,700 times, h-index = 56. Mainly engaged in the research of fuel cells, electrocatalysis, water treatment and other fields.
Shao Zongping is a professor and doctoral supervisor of the State Key Laboratory of Materials Chemical Engineering, School of Chemical Engineering, Nanjing University of Technology. The “National Ten Million Talent Project” has outstanding contributions from young experts, Yangtze River scholars, the National Jieqing, and the Ten Thousand Talents Program. He has published more than 600 SCI papers in international journals such as Nature, Nature Energy, Nature Communications, and Science Advances; these papers have been cited more than 28700 times, h-index = 80. Mainly engaged in the research of fuel cells, solar cells, photocatalysis, electrocatalysis, lithium / sodium ion batteries, water treatment and other fields.
This article was compiled and contributed by Microworld.
Source-WeChat public account: Source

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd 京ICP备16054715-2号 |