
hotline:
17715390137
Tel/Wechat:
18101240246 (Technology)
0512-68565571
Email:mxenes@163.com (Sales Engineer)bkxc.bonnie@gmail.com
Scan the code to follow or search the official account on WeChat:
2D Materials Fronrier After paying attention,
click on the lower right corner to contact us,
Enter enterprise WeChat.
Professional Services Online

已传文件:photo/2020410112158870.png
Foreword It is
well known that ammonia plays an important role in the development of agriculture and industry. The traditional ammonia preparation relies on the Haber-Bosch synthesis method, which consumes a lot of energy and causes serious environmental pollution. Therefore, other electrocatalysts for electrochemical reduction reactions have emerged, using water as a source of hydrogen to reduce nitrogen to ammonia. Methods of ammonia synthesis by electrochemical reduction (Figure 1), such as photocatalytic ammonia production, electrocatalytic ammonia production, and photocatalytic ammonia production [ 1] . Among them, electrocatalytic reduction of ammonia to ammonia has attracted more and more attention due to its low cost and controllable conditions, showing the development trend of blowout.
FIG . Three new ways ammonia [ 1] .
development path
Figure 2. NRR response mechanism [ 3] .
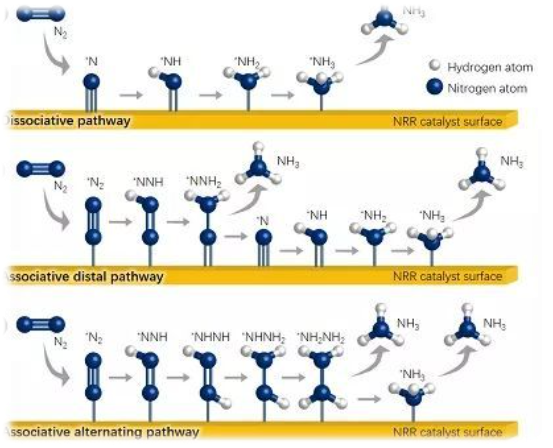
Then, the activation of nitrogen molecules requires the surface of the catalyst to be able to adsorb N 2. On the other hand, the catalyst needs to have higher selectivity and compete with the strong hydrogen evolution reaction. Then, it is necessary to develop a side reaction that can inhibit hydrogen evolution and limit the diffusion of protons and electrons to the catalyst surface. From the theoretical calculation of DFT, in order to obtain high NRR selectivity, the side reaction HER can be suppressed by controlling the concentration and activity of protons. From the kinetic point of view, the reaction NRR proton concentration is less likely to be limited, unless certain low concentrations, then the amount of proton mass transport to the electrode is not sufficient to match the rate NRR [2 - . 4 ] . In order to control the supply of proton sources, an aprotic electrolyte added with an appropriate amount of water, acid or alkali can be used. For now, Fe, W, Mo, Ru are recognized as the most efficient NRR catalysts.
Electrocatalyst performance comparison
Table 1 . Summarizes the different electrical properties of Comparative Catalyst [ 5,6] .
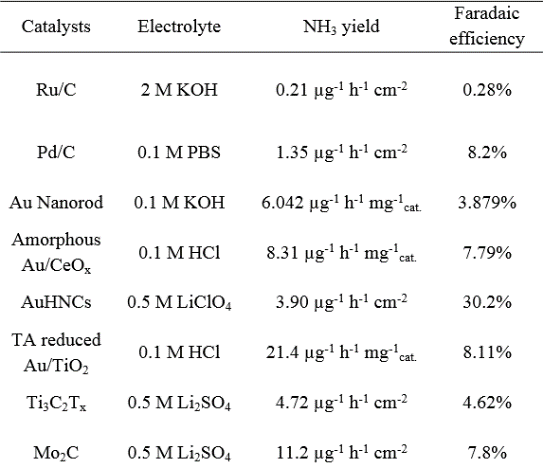
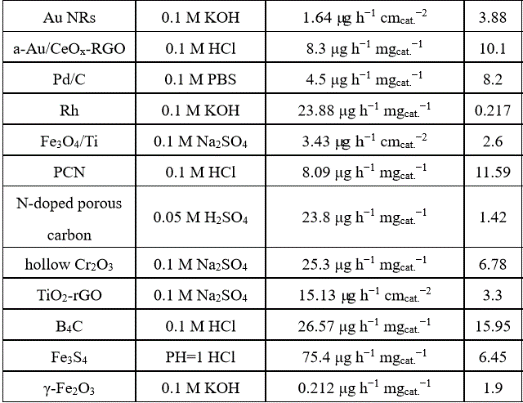
From the data in Table 1, the Ru and Fe-based catalysts have higher ammonia production rates and Faraday efficiency, and the precious metal Au also has good catalytic activity for NRR. In addition, the rare earth element Ce also started to show a good trend in this field. However, it is difficult for the catalyst‘s ammonia production rate and Faraday efficiency to have optimal performance at the same time. The problem to be solved urgently is how to take into account the relationship between the two at the same time, suppress the competitive response HER, and reduce the NRR overpotential. The catalyst can achieve high electro-catalytic NRR selectivity, and the competitive reaction-hydrogen evolution reaction can be suppressed by controlling the activity and concentration of protons. In terms of selectivity and rate of catalysts, most traditional catalysts choose transition metals with better hydrogen evolution reaction activity. Can other metal-based materials used in electrocatalytic reactions, such as carbon dioxide reduction, also be used in electrocatalytic nitrogen reduction? First of all, from the theoretical calculation point of view (see Figure 3 below for free energy calculation), Fe, W, Mo, Ru and other metals all meet this condition (as seen in the green area in the upper left corner of Figure 3).
FIG 3 . N and H on the opposite surfaces of the metal adsorption energy surface coating theory [ 3] .
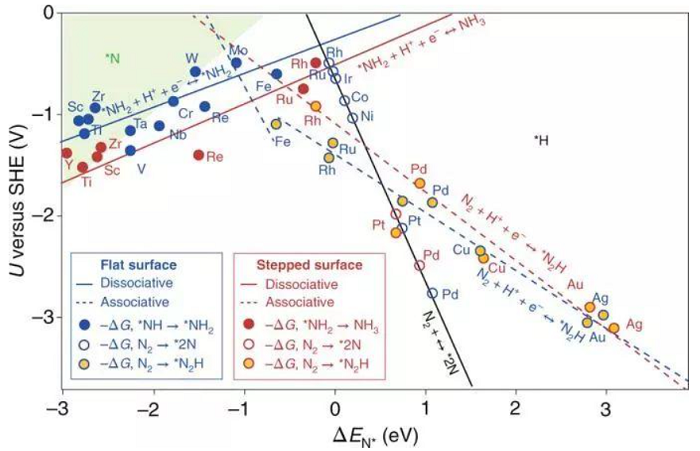
Do other metals, such as bismuth-based materials used in photocatalysis, also satisfy this condition?
Table 2. Summary of the application of Bi-based catalysts in NRR
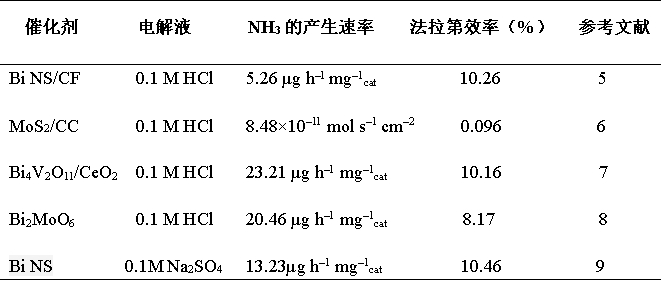
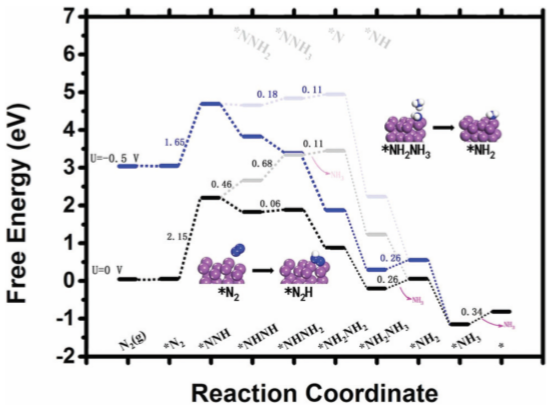
The aggregated data shows that the bismuth-based materials also have good selectivity, catalytic efficiency and rate for the NRR reaction. From the theoretical calculation, Bi NS / CF (Bi nanosheets are arranged on Cu foil). Figure 4 is the theoretically calculated free energy distribution diagram, and the reaction occurs on the Bi (012) surface.
Figure 4. Free energy distribution of Bi NS / CF under different NRR applied potentials, the asterisk (*) indicates the adsorption site [ 5]
In addition, the NRR catalytic performance of Bi nanosheets (Bi NS) without base foam copper was examined. Figure 5d shows that bismuth atoms can effectively provide p-orbital electrons, thereby activating nitrogen molecules. Further analysis by X-ray fine absorption spectroscopy shows that the shortening of the interlayer Bi-Bi bond length can effectively promote the delocalization of the p orbital electrons of Bi, thereby enhancing its adsorption and activation of N 2 .
Figure 5. Free energy distribution of Bi NS under different applied potentials of NRR. The asterisk (*) indicates the adsorption site [ 9] .
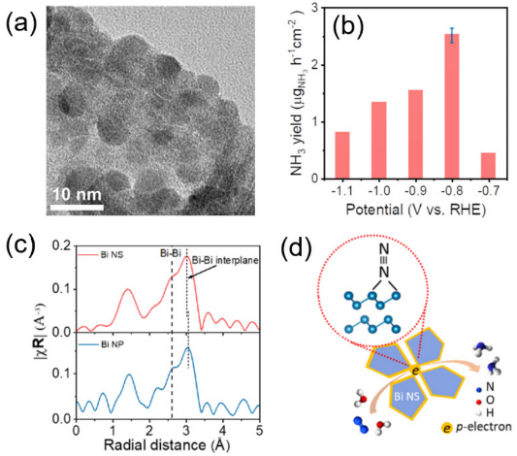
Figure 6. Scanned pictures of Bi NS before (a) and after (b) NRR process [ 9] .
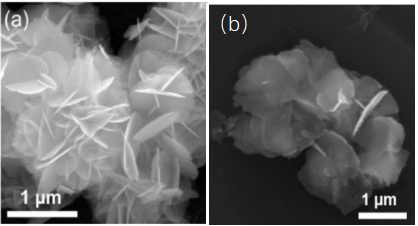
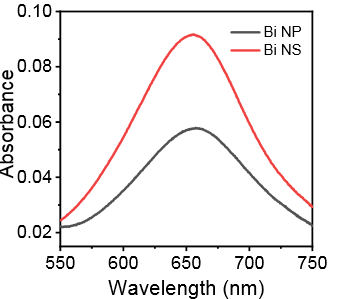
After the NRR test, the Bi nanosheets can still maintain the previous morphology (Figure 6), indicating that the catalyst has excellent nitrogen reduction performance by in-situ electrochemical reduction. Compared with Bi nanoparticles (Bi NP), it can expose more edge active sites and improve the selectivity of the catalytic reaction. Figure 7 compares the UV absorption spectra of Bi NP and Bi NS after NRR electrolysis, and directly concludes that bismuth nanoplates have better catalytic ammonia production performance than bismuth nanoparticles.
Figure 7. UV-vis spectra of Bi NS and Bi NP [ 9] .
Stability and turnover frequency stability
Figure 8. Bi 2 MoO 6 / CP electrolysis in N 2 saturated solution, time and current density curve for 24 hours voltage (–0.6 V) [ 8]
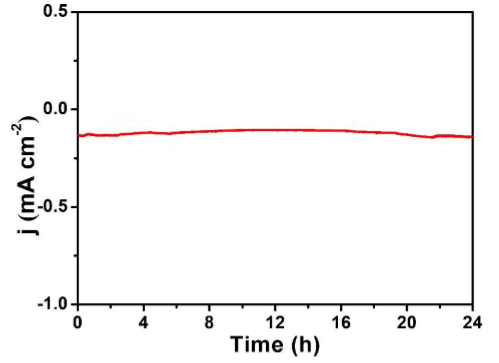
The stability test time of the electrocatalyst is at least more than 5 hours, and it has continuous activity. Turnover Frequency In addition, another important indicator in this field is that the turnover frequency of the catalyst needs to be greater than 1. to sum up
Electrocatalytic nitrogen reduction is a sustainable ammonia synthesis process. Its catalyst design depends on the electrode / catalyst / electrolyte combination. For how to construct an excellent catalyst, the structure of electrocatalytic nitrogen reduction catalyst, or the combination of catalyst and electrolyte is also a way to improve the catalytic selectivity. For energy efficiency, focusing on the characteristics of the catalyst is a key strategy. Through the construction of defect engineering, the modification of the catalyst surface, and the provision of low overpotential steps, research in theoretical mechanics is the key to determining these possibilities, especially in the search for low Specific surface features of the energy mechanism. In addition, for the unified data report, the rate of ammonia production should use a unified quantification standard, and the detection method should be standardized and unified. The road to ammonia synthesis is long and full of hope! References [1] Li, M., Huang, H., Low, J., Gao, C., Long, R., & Xiong, Y. Small Methods, 2018 , 1800388. [2] D. Bao, Q . Zhang, F. Meng, H. Zhong, M. Shi, Y. Zhang, J. Yan, Q. Jiang and X. Zhang , Adv. Mater., 2017 , 29, 1604799. [3] SL Foster, SIP Bakovic , RD Duda, S. Maheshwari, RD Milton, SD Minteer, MJ Janik, JN Renner and LF Greenlee, Nat. Catal., 2018, 1, 490–500.[4] L. Wang, MK Xia, H. Wang, KF Huang, CX Qian, CT Maravelias and GA Ozin, Joule, 2018, 2, 1055–1074. [5] R. Zhang, L. Ji, W. Kong , HB Wang, R. Zhao, H. Chen, T. Li, B. Li, Chem. Commun., 2019, 55, 5263—5266. [6] L. Zhang, X. Ji, X. Ren, Y. Ma, X. Shi, Z. Tian, AM Asiri, L. Chen, B. Tang and X. Sun, Adv. Mater., 2018, 30, 1800191. [7] C. Lv, C. Yan, G. Chen , Y. Ding, J. Sun, Y. Zhou and G. Yu, Angew. Chem., Int. Ed., 2018, 57, 6073–6076. [8] Z. Xing, WH Kong, TW Wu, H. Xie, T. Wang, Y. Luo, X. Shi, and XP Sun. ACS Sustainable Chem. Eng., 2019, 7, 12692−12696. [9] LQ Li, C. Tang, BQ Xia, H. Jin, Y. Zheng and SZ Qiao, ACS Catal ., 2019 , 9 , 2902.
Source of information:

| Reminder: Beijing Beike New Material Technology Co., Ltd. supplies products only for scientific research, not for humans |
| All rights reserved © 2019 beijing beike new material Technology Co., Ltd 京ICP备16054715-2号 |