已传文件:photo/202037132813461.png
In the field of catalysis, molecular sieves belong to the classic materials that must be mentioned. The use of molecular sieve can be traced back to 200 years ago. Beginning in the 1960s, molecular sieve catalysts have played an important role in petrochemicals. While some people are constantly chasing the forefront, some people need to review the classics and discover new science in the old topics of molecular sieve.
Recently, Science Magazine has just published two articles in succession, and JACS has published four articles in the past two days, explaining how to discover new discoveries in the old topic of molecular sieve. Let ’s take a look first. What are the latest 4 JACS and 2 Science studies?
In the latest 4 articles of JACS, one is based on the synthesis of ultra-stable zeolite, the other is about the effect of structure directing agent on the structure of zeolite, and the other is the use of zeolite molecular sieve as the encapsulation structure to achieve the high performance of atomic dispersion catalyst Catalysis; the last article found that [InH2] + ion is a potential catalytic active site, which can efficiently catalyze the non-oxidative dehydrogenation of ethane.
1. JACS: Collaborative and competitive occlusion of organic and inorganic structure directors in chabazite will affect its aluminum alignment
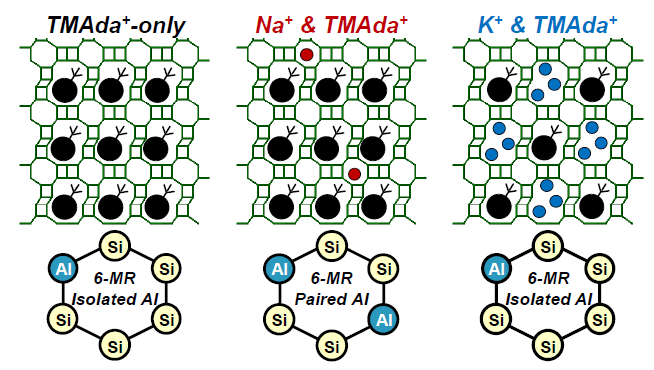
Zeolite molecular sieves have a clear crystal framework structure, which is conducive to linking data and insights from experimental and theoretical evaluations of material structure and function, and provides a convenient platform for the rational and clever design of materials. Recently, Professor Rajamani Gounder‘s group at Purdue University and Professor William F. Schneider‘s group at the University of Notre Dame combined experiments and theory to explore the micropores of organic and inorganic structure directing agent (SDA) Cooperative or competitive effects occupied in the voids, and rationalization of SDA site selection to skew the Al framework (Al-O (-Si-O) x-Al, x) between CHA zeolites with basically fixed composition (Si / Al = 15) = 1-3) The effect of permutation.
Highlights of this article:
1) CHA molecular sieve crystallized from a mixture of TMAda + and Na + contains a TMAda + in each cage, and the content of Na + co-adsorbed is linearly proportional to the number of 6-MR paired Al sites, and quantified by Co2 + titration. In contrast, CHA zeolite crystallized using a mixture of TMAda + and K + provided evidence that, on average, three K + cations replaced one TMAda + occupying a cage and mainly contained 6-MR separated Al sites. Furthermore, CHA is crystallized from a synthetic medium containing K + in an inorganic / organic ratio ten times higher than Na + before forming a competitive crystalline phase, thereby providing a way to reduce the amount of organic SDA required to crystallize high silica CHA.
2) Density functional calculations show that the differences in Na + and K + ionic radii determine their preference for positions in different CHA rings, which affects their ability to occlude and stabilize the energy of different Al configurations with TMAda +. The Monte Carlo model confirmed that the energy difference caused by Na + or K + co-blocking promoted the formation of 6-MR and 8-MR paired Al permutations, respectively.
3) These results highlight the opportunity to develop a mixture of organic and inorganic SDA during the crystallization of zeolite, in order to make more efficient use of organic SDA and affect the arrangement of the framework Al.
references:
John R. Di Iorio et al. Cooperative and Competitive Occlusion of Organic and Inorganic Structure Directing Agents within Chabazite Zeolites Influences Their Aluminum Arrangement. J. Am. Chem. Soc., 2020.
DOI: 10.1021 / jacs.9b13817
https://pubs.acs.org/doi/10.1021/jacs.9b13817
2. JACS: Synthesis of Ultra-Stable Molecular Sieves by Designing Liquid-Mediated Processing
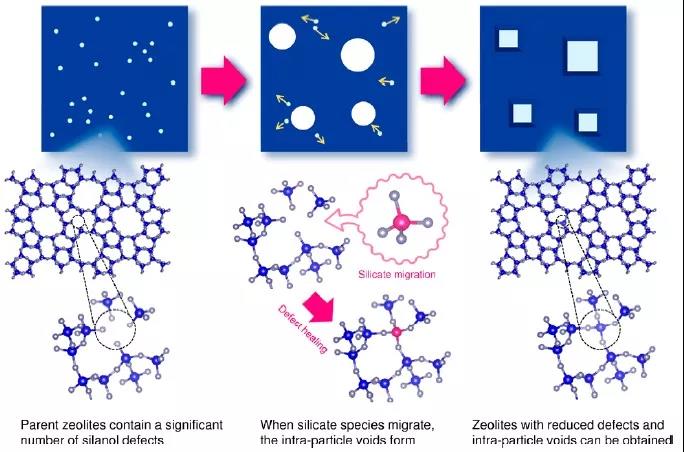
At present, improving the stability of porous materials in practical applications is still a huge challenge. Aluminosilicate zeolites are sometimes exposed to high temperature steam (1000 ° C) when used for adsorption and catalysis. Defective sites in the framework of the siliceous zeolite molecular sieve will lead to the degradation of the material, so a feasible method for repairing the defect is urgently needed. Recently, Professor Toru Wakihara‘s group at the University of Tokyo in Japan proposed a method to synthesize super-stable high-silica zeolite molecular sieves by repairing defect sites.
Highlights of this article:
1) Method for reducing defect sites by using liquid-mediated processing without using other silylating agents, high silica (SiO2 / Al2O3) with * BEA-, MFI- and MOR-type topologies > 240) The stability of zeolite can be significantly improved.
2) When exposed to steam at extremely high temperatures (900-1150 ° C), the stabilized zeolite can still maintain its crystallinity and microporous structure, while the parent commercial zeolite is completely degraded.
3) The self-defect repair method proposed in this paper provides a new way for species to migrate through porous bodies, and greatly improves the practicality of zeolite in harsh environments.
references:
Kenta Iyoki et al. Extremely Stable Zeolites Developed via Designed Liquid-Mediated Treatment. J. Am. Chem. Soc., 2020.
DOI: 10.1021 / jacs.9b12709
https://dx.doi.org/10.1021/jacs.9b12709

3. JACS: Atomically accurate heterogeneous catalyst, sandwich core-shell structure works wonders!
Heterogeneous catalysts with precise atomic interface structure can not only ensure excellent catalytic activity, but also provide models for understanding the structure-activity relationship of catalysts. Therefore, they have gradually attracted widespread attention in the field of catalysis. In view of this, Zhu Manzhou, Sheng Hongting from Anhui University, and Didier Astruc of Bordeaux, France jointly proposed a new strategy for the preparation of heterogeneous catalysts with precise atomic interface structure.
Highlights of this article:
1) The research team successfully prepared a ZIF-8 @ Au25 @ ZIF-67 composite structure with a sandwich structure through a coordination-induced self-assembly strategy. The composite structure has a precise atomic interface structure and can be used as a heterogeneous catalyst.
2) The research found that the composite catalyst has superior catalytic performance in 4-nitrobenzene hydrogenation and terminal alkyne carbonylation reactions. In the 4-nitrobenzene hydrogenation reaction, the TOF value of the catalyst is as high as 588 min-1. In the terminal alkyne carbonylation reaction, the activity of the catalyst is as high as 99%.
3) Subsequent research found that the performance of the composite catalyst showed a volcanic curve with the shell thickness, and the catalytic effect was best when the shell thickness was 12 nm.

references:
Yapei Yun, et al. Design and Remarkable Efficiency of the Robust Sandwich Cluster Composite Nanocatalysts ZIF-8 @ Au25 @ ZIF-67, J. Am. Chem. Soc. 2020.
DOI: 10.1021 / jacs.0c00378
https://pubs.acs.org/doi/10.1021/jacs.0c00378
4. JACS: Atomically accurate heterogeneous catalyst, sandwich core-shell structure works wonders!
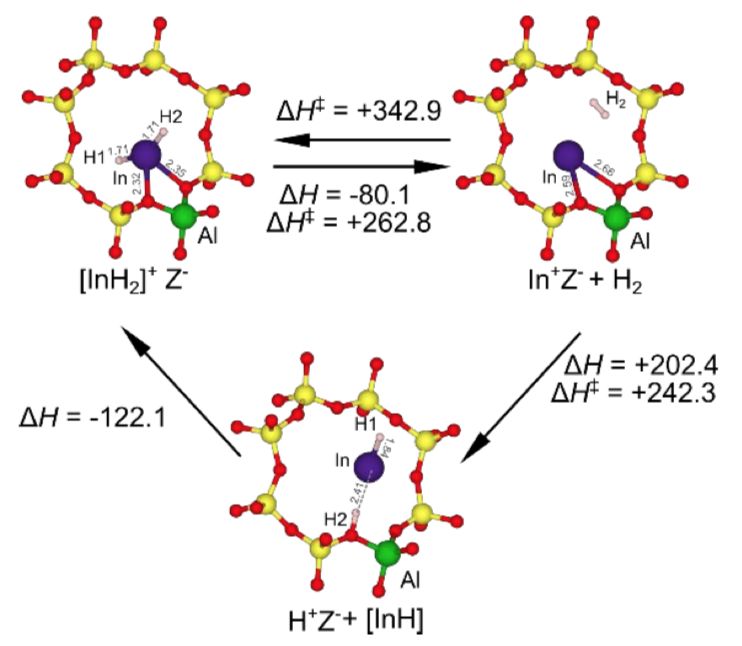
The hydrides on the solid surface can participate in heterogeneous catalyzed hydrogenation, dehydrogenation and hydrogenation reactions. The hydride on the metal surface is highly fluid, while the hydride on the metal oxide surface is relatively stable. Some metal oxides and supported metal nanoparticles have the potential to promote efficient hydrogenation, dehydrogenation, and deoxygenation, and surface metal hydrides are considered key intermediates. However, due to the complexity of the solid surface and the difficulty of solid structure analysis, studying the formation, characterization, and performance of surface hydrides on solid materials is still challenging. In addition, in terms of catalysis, the surface metal hydride often participates in the reaction as a transient and / or unstable intermediate, so the details of its structure and catalytic function remain unclear. In view of this, Ken-ichi Shimizu and Zen Maeno of Hokkaido University have co-synthesized an indium (In) -hydride supported by CHA zeolite. Mechanism studies based on kinetics, in-situ spectroscopy, and theoretical studies show that [InH2] The + ion is a potential catalytic active site, which can efficiently catalyze the non-oxidative dehydrogenation of ethane. This work used zeolite as a carrier to synthesize isolated surface hydrides, which promoted the synthesis of surface hydrides and their catalytic development.
Highlights of this article:
1) They calculated the in-situ Fourier transform infrared spectroscopy (FTIR), XAFS spectroscopy and density functional theory (DFT) to study the formation and detailed structure of hydride in CHA zeolites. At high temperature (> 773 K), H2 treated In-exchanged CHA zeolite (In-CHA) to form In-hydride. [InH2] + ion at the anion site of the framework is a reasonable structure.
2) Compared with Ga and Zn-exchanged CHA molecular sieves, In-CHA exhibits high selectivity and excellent catalytic activity for the non-oxidative dehydrogenation of ethane, and has good catalytic stability, which can be stable for at least 90 h. . In addition, the [InH2] + ion in In-CHA can be used as a catalytic active site for selective dehydrogenation.
Zen Maeno, et al. Isolated Indium-hydrides in CHA Zeolites: Speciation and Catalysis for Non-oxidative Dehydrogenation of Ethane. Journal of the American Chemical Society, 2020.
DOI: 10.1021 / jacs.9b13865
https://doi.org/10.1021/jacs.9b13865
In the latest two Science this year, one work is to creatively design and propose a "molecular fence" based on molecular sieve catalysts. The other work is to use the pore structure and gas separation characteristics of molecular sieves for Providing more pure raw materials is not unique.

5. Science: Molecular Fence Strategy to Improve Methanol Oxidation to Methanol Production
The direct oxidation of methane to methanol has always been a "Dream Reaction" in catalytic chemistry. Compared with gas-phase methane conversion, the energy consumption of the methane oxidation reaction in a liquid solvent is lower at a mild temperature, but a strong oxidant such as selenic acid is required, which will generate a large number of highly polluting by-products. To solve this problem, an environmentally friendly oxidant, hydrogen peroxide (H2O2), was used to perform methane oxidation without any toxic salts and strong acids. However, compared to gaseous O2, the cost of H2O2 is still relatively expensive.
On January 10, 2020, Wang Liang and Professor Xiaoshou Xiao from Zhejiang University and others proposed the concept of molecular fencing. By fixing AuPd alloy nanoparticles in aluminosilicate zeolite crystals, and then modifying the outer surface of the zeolite with organosilane, the design and preparation A heterogeneous catalyst for increasing the yield of methanol in methane oxidation by generating hydrogen peroxide in situ at a mild temperature (70 ° C) is provided.
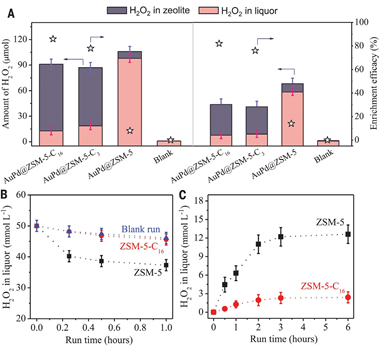
Silane can diffuse hydrogen, oxygen, and methane to the active site of the catalyst, and at the same time limit the generated hydrogen peroxide near the active site to increase the local enrichment concentration, which greatly improves the reaction efficiency and selectivity, and the methane conversion rate is up to 17.3% At this time, the selectivity of methanol can reach 92%, which is equivalent to a methanol productivity of 91.6 millimoles per gram of AuPd per hour, which is the highest level currently. This work directly applies methane to produce valuable products, and the molecular barrier concept opens a new production path for the production of highly efficient methane partial oxidation catalysts.
references:
Zhu Jin, et al. Hydrophobic zeolite modification for insitu peroxide formation in methane oxidation to methanol. Science, 2020.
DOI: 10.1126 / science.aaw1108
https://science.sciencemag.org/content/367/6474/193?rss=1

Silane can diffuse hydrogen, oxygen, and methane to the active site of the catalyst, and at the same time limit the generated hydrogen peroxide near the active site to increase the local enrichment concentration, which greatly improves the reaction efficiency and selectivity, and the methane conversion rate is up to 17.3% At this time, the selectivity of methanol can reach 92%, which is equivalent to a methanol productivity of 91.6 millimoles per gram of AuPd per hour, which is the highest level currently. This work directly applies methane to produce valuable products, and the molecular barrier concept opens a new production path for the production of highly efficient methane partial oxidation catalysts.
references:
Zhu Jin, et al. Hydrophobic zeolite modification for insitu peroxide formation in methane oxidation to methanol. Science, 2020.
DOI: 10.1126 / science.aaw1108
https://science.sciencemag.org/content/367/6474/193?rss=1

references:
Huazheng Li et al. Na + -gatedwater-conducting nanochannels for boosting CO2 conversion to liquid fuels. Science 2020, 367, 667-671.
https://science.sciencemag.org/content/367/6478/667












